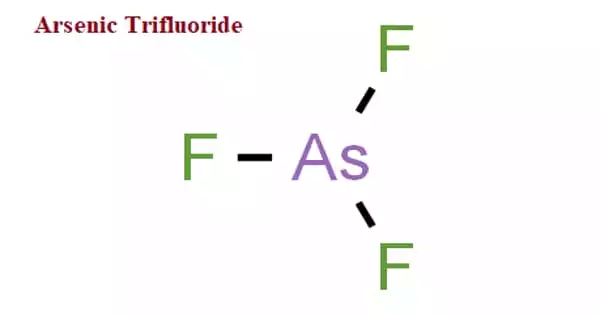
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the formula AsF3. It is a colorless liquid that easily reacts with water. It is used to fluorinate non-metal chlorides to fluorides, as a fluorinating reagent, catalyst, dopant, and ion implantation source.
Arsenic trifluoride is a fluoride of arsenic. Arsenic is a chemical element with the symbol ‘As’ and the atomic number 33. It is a deadly metalloid with numerous allotropic forms, including yellow (molecular non-metallic) and multiple black and grey forms (metalloids). Arsenic is found in three metalloidal forms with various crystal shapes in nature (the minerals arsenopyrite and the considerably rarer arsenolamprite and pararsenolamprite), although it is more usually found as a combination with other elements.
Properties
It is a colorless oily liquid; fumes in air; etches glass; density 2.666 g/ml at 0°C; boils at 60.4°C; vapor pressure 100 torr at 13.2°C; solidifies at -8.5°C; decomposes in water; soluble in alcohol, ether, benzene and ammonia solution.
- Chemical formula: AsF3
- Molar mass: 131.9168 g/mol
- Appearance: colorless oily liquid
- Density: 2.666 g/cm3 (0 °C)
- Melting point: −8.5 °C (16.7 °F; 264.6 K)
- Boiling point: 60.4 °C (140.7 °F; 333.5 K)
- Solubility in water: decomposes
- Solubility: soluble in alcohol, ether, benzene and ammonia solution
Preparation
It is made by combining hydrogen fluoride and arsenic trioxide. In the gas phase, it possesses a pyramidal molecular structure, which is also present in the solid phase. Arsenic trioxide is combined with fluorosulfonic acid to produce the chemical. It can also be made by reacting arsenic trioxide with a solution of sulfuric acid and calcium fluoride.
It can be prepared by reacting hydrogen fluoride, HF, with arsenic trioxide:
6HF + As2O3 → 2AsF3 + 3H2O
It has a pyramidal molecular structure in the gas phase which is also present in the solid. In the gas phase the As-F bond length is 170.6 pm and the F-As-F bond angle 96.2°.
Arsenic trifluoride is used as a fluorinating agent for the conversion of non-metal chlorides to fluorides, in this respect it is less reactive than SbF3.
Salts containing AsF4− anion, such as CsAsF4, can be produced. The potassium salt KAs2F7, formed from KF and AsF3, contains AsF4− and AsF3 molecules, as well as evidence of interaction between the AsF3 molecule and the anion.
SbF5 reacts with AsF3. The resulting ionic compound might be characterized as AsF2+ SbF6−. The authors conclude, however, that the produced product cannot be understood either as an ionic compound or solely as the neutral adduct AsF3SbF5. The crystal structure exhibits properties of both an ionic pair and a neutral adduct, occupying a middle ground between the two concepts.