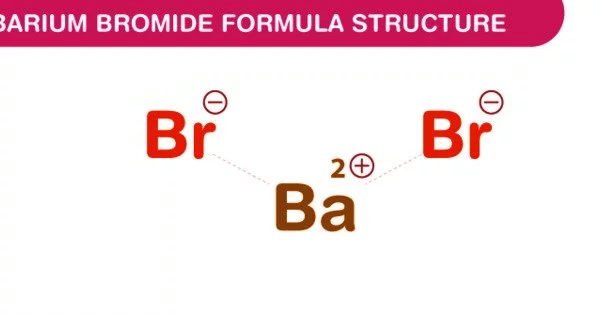
The chemical compound with the formula BaBr2 is barium bromide. It is a significant chemical compound having the chemical formula Barium bromide. It is also known as barium (2+) dibromide or anhydrous barium bromide. It appears as a white solid that is water soluble but poisonous in aqueous form. It dissolves well in water and is hazardous, like barium chloride.
Barium bromide anhydrous, along with a few other water-soluble barium compounds, is poisonous and produces severe poisoning when consumed. It is made by combining hydrobromic acid with barium sulfide or barium carbonate.
Properties
Barium bromide is a colorless solid that is soluble in water. The precise mass of barium bromide is 297.74 g/mol, and the monoisotopic mass is 297.742 g/mol. The number of hydrogen bond donors is zero, while the number of hydrogen bond acceptors is two. The compound is canonicalized and contains three covalently bonded components.
- Chemical formula: BaBr2 (anhydrous); BaBr2·2H2O (dihydrate)
- Molar mass: 297.14 g/mol
- Appearance: White solid
- Density: 4.78 g/cm3 (anhydrous); 3.58 g/cm3 (dihydrate)
- Melting point: 857 °C (1,575 °F; 1,130 K)
- Boiling point: 1,835 °C (3,335 °F; 2,108 K)
- Solubility in water: 92.2 g/100 mL (0°C)
- Magnetic susceptibility (χ): -92.0·10−6 cm3/mol

It is a white solid that is hygroscopic. Due to the presence of Barium ions, it produces a yellow-greenish color in the flame test. The most common application for barium bromide is in photography. This compound does not occur naturally; rather, it is synthesized from barium sulphide, barium carbonate, and hydrobromic acid.
Preparation
Barium bromide can be prepared by treating barium sulfide or barium carbonate with hydrobromic acid:
BaS + 2 HBr → BaBr2 + H2S
BaCO3 + 2 HBr → BaBr2 + CO2 + H2O
Barium bromide crystallizes from concentrated aqueous solution in its dihydrate , BaBr2·2H2O. Heating this dihydrate to 120 °C gives the anhydrous salt.
Uses
Barium bromide is a precursor to chemicals used in photography and to other bromides.
- It is used as a precursor in chemicals.
- Used in photography.
- It was used in a process called fractional crystallization to purify radium.
- Used in the manufacturing of other bromides.
- Used in the production of phosphors.
Historically, barium bromide was used to purify radium in a fractional crystallization procedure pioneered by Marie Curie. Because radium preferentially precipitates in a solution of barium bromide, the ratio of radium to barium in the precipitate would be greater than the ratio in the solution.
Safety
Barium bromide, like other water-soluble barium salts, is hazardous. Barium bromide anhydrous, along with a few other water-soluble barium salts, is poisonous and, if consumed, produces serious poisoning.