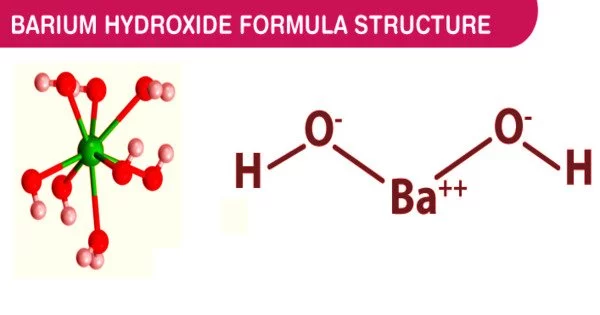
Barium hydroxide is a chemical compound with the formula Ba(OH)2. It is a clear white powder with no odor. It is poisonous in nature. One of the main barium compounds is the monohydrate (x = 1), also known as baryta or baryta-water. The most common commercial form is this white granular monohydrate.
Barium hydroxide is ionic in nature; for example, Ba(OH)2 in aqueous solution can provide two hydroxide ions per molecule. It forms a strong caustic base in aqueous solution. It has many applications, including sulphide testing, pesticides, and the production of alkali and glass.
Properties
The melting point of Barium Hydroxide differs with the amount of water in it. The octahydrate form of Barium oxide melts at 78-degree celsius. The monohydrate form melts at 3000 Celsius and the anhydrous form melts at 4070 celsius. It boils at 7800 celsius. If you keep on boiling and the temperature reaches 8000 celsius, it will decompose and give out Barium Oxide.
- Chemical formula: Ba(OH)2
- Molar mass: 171.34 g/mol (anhydrous); 189.355 g/mol (monohydrate); 315.46 g/mol (octahydrate)
- Appearance: white solid
- Density: 3.743 g/cm3 (monohydrate); 2.18 g/cm3 (octahydrate, 16 °C)
- Melting point: 78 °C (172 °F; 351 K) (octahydrate); 300 °C (monohydrate)
- 407 °C (anhydrous)
- Boiling point: 780 °C (1,440 °F; 1,050 K)

Preparation and structure
Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water:
BaO + H2O → Ba(OH)2
When heated in air, it crystallizes as the octahydrate, which then converts to a monohydrate. The monohydrate will yield BaO and water in a vacuum at 100 °C. The monohydrate is layered in structure (see picture above). The anti-prismatic geometry of the Ba2+ centers is square. Each Ba2+ center is bound by two water ligands and six hydroxide ligands that bridge to neighboring Ba2+ center sites twice and three times. Individual Ba2+ centers in the octahydrate are eight coordinate but do not share ligands.
Reactions
Barium hydroxide decomposes to barium oxide when heated to 800 °C. Barium carbonate is formed as a result of the reaction with carbon dioxide. Because it is a strong base, its highly alkaline aqueous solution is neutralized by acids. It is especially useful in reactions requiring weak organic acid titrations. It generates barium sulfate and barium phosphate from sulfuric and phosphoric acids, respectively. By reacting with hydrogen sulfide, barium sulfide is formed. A double replacement reaction may occur when a barium hydroxide aqueous solution is mixed with many solutions of other metal salts, resulting in the precipitation of many insoluble or less soluble barium salts.
Barium hydroxide reactions with ammonium salts are strongly endothermic. The reaction of barium hydroxide octahydrate with ammonium chloride or ammonium thiocyanate is a popular classroom chemistry demonstration because it produces temperatures cold enough to freeze water and enough water to dissolve the resulting mixture.
Uses
Barium hydroxide is used as a precursor to other barium compounds in industry. The monohydrate is used in the dehydration and removal of sulfate from a variety of products. This application takes advantage of barium sulfate’s extremely low solubility. This industrial application is also used in the laboratory.
Laboratory uses
In analytical chemistry, barium hydroxide is used to titrate weak acids, particularly organic acids. Because barium carbonate is insoluble in water, its clear aqueous solution is guaranteed to be carbonate-free, unlike those of sodium hydroxide and potassium hydroxide. This enables the use of indicators like phenolphthalein or thymolphthalein (with alkaline color changes) without the risk of titration errors caused by the presence of carbonate ions, which are much less basic.
Safety
Barium hydroxide, like the other strong bases and water-soluble barium compounds, is corrosive and toxic, causing skin irritation and burns as well as eye damage. Inhaling barium dusts can cause nasal and upper respiratory tract irritation, as well as a benign pneumoconiosis known as baritosis.