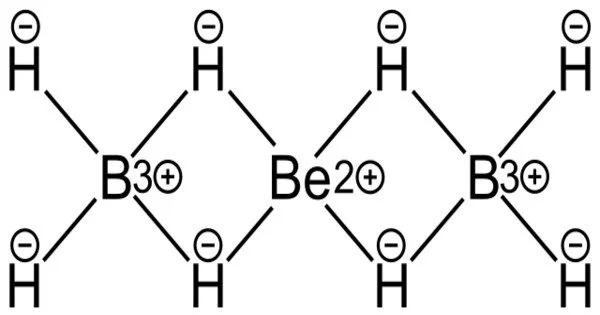
Beryllium borohydride is an inorganic compound with the chemical formula Be(BH4)2. Previously, scientists proposed numerous different arrangements for the monomeric boronberylliumboron bonds in the gas phase, and the number and position of hydrogen atoms in the structure were also unknown. Furthermore, solid beryllium borohydride is known to exist as polymers forming a helix, adding to the confusion about the individual structures.
Beryllium Borohydride has an extremely high total hydrogen storage capacity. Originally, Be(BH4)2 was made by reacting BeMe2 with diborane and, more conveniently, by mechanochemical exchange response of BeCl2 with alkali metal borohydrides followed by vacuum distillation at 140 °C.
Properties
Beryllium Borohydride is very unstable, exploding on contact with air and moisture. Unfortunately, the intense toxicity of beryllium, and very high reactivity of Be(BH4)2, makes this material inappropriate for hydrogen storage despite low decomposition temperature and high frequency.
- Chemical formula: Be(BH4)2
- Molar mass: 38.70 g/mol
- Appearance: white crystals
- Density: 0.604 g/cm3
- Melting point: 91.3 °C (196.3 °F; 364.4 K)
- Boiling point: 123 °C (253 °F; 396 K) decomposes
- Solubility in water: reacts
- Solubility: soluble in benzene, diethyl ether
- Crystal structure: tetragonal
Structure
The crystal structure is made up of a helical polymer of BH4Be and BH4 structure units.
The new beryllium borohydride molecule, which is stabilized by a carbene ligand, has a five-coordinate beryllium arranged in a distorted square pyramid. Beryllium borohydrides are known to be highly reactive, even explosive compounds, but Robinson’s molecule is stable for several days on the benchtop. This first unequivocal crystal structure advances chemical understanding of these types of compounds, paving the way for future development of new systems for hydrogen storage and alternative fuel technology.
Production
Beryllium borohydride is formed when beryllium hydride reacts with diborane in an ether solution.
Beryllium borohydride compounds have long piqued the interest of alternative energy researchers due to their potential for hydrogen gas storage, as they have the highest hydrogen capacity of any metal borohydride. However, the structure of these compounds, and thus how they would store hydrogen, has been a source of consternation for over 70 years.
Application
The purest beryllium hydride is obtained by the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:
Be(BH4)2 + 2 PPh3 → 2 Ph3PBH3 + BeH2