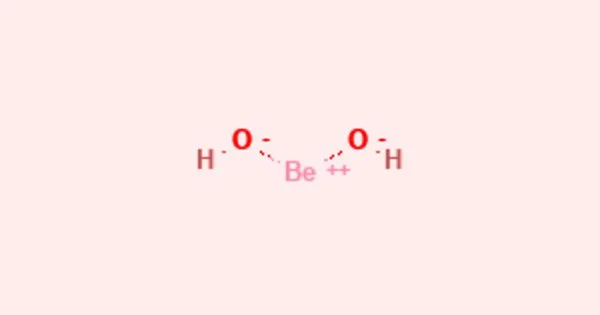
Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide that dissolves in acids as well as alkalis. It is a metal hydroxide with amphoteric properties that is also known as hydrated beryllia and beryllium dihydroxide (behaves both as an acid and a base). It is produced industrially as a byproduct of the extraction of beryllium metal from the ores beryl and bertrandite.
Pure beryllium hydroxide is found in nature as the rare orthorhombic mineral behoite and the even rarer monoclinic clinobehoite. Natural pure beryllium hydroxide is either scarce (in the form of the mineral behoite, orthorhombic) or extremely scarce (clinobehoite, monoclinic). The α-form (a gel) is formed when alkali is added to beryllium salt solutions. The rhombic β-form precipitates when this is left to stand or boiled. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.
Properties
- Chemical formula: BeH2O2
- Molar mass: 43.026 g·mol−1
- Appearance: Vivid white, opaque crystals
- Density: 1.92 g cm−3
- Melting point: (decomposes)
- Solubility in water: 0.0000023965 g/L
- Solubility product (Ksp): 6.92×10−22
- Acidity (pKa): 3.7
- Molecular shape: Linear

Reactions
Beryllium hydroxide is difficult to dissolve in water. With alkalis it dissolves to form the tetrahydroxoberyllate/tetrahydroxidoberyllate anion, [Be(OH)4]2–. With sodium hydroxide solution: 2NaOH(aq) + Be(OH)2(s) → Na2[Be(OH)4](aq)
With acids, beryllium salts are formed. For example, with sulfuric acid, H2SO4, beryllium sulfate is formed:
Be(OH)2 + H2SO4 → BeSO4 + 2H2O
Beryllium hydroxide dehydrates at 400 °C to form the soluble white powder, beryllium oxide:
Be(OH)2 → BeO + H2O
Further heating at higher temperature produces acid insoluble BeO.
Preparation
Pure beryllium hydroxide is produced as a byproduct of beryllium extraction from mineral ore beryl (Be3Al2Si6O18) or bertrandite (Be4Si2H2O9). To produce a water-soluble sulfate, the ore is melted, solidified, crushed, and then treated with sulfuric acid. The sulfate is subjected to several chemical extraction procedures in order to remove all contaminants and form beryllium hydroxide as a precipitate.
Use
- As a source in the manufacture of beryllium and its oxide.
- In braking systems, engines, altimeters, and precision tools in the aerospace industry.
- Producing anti-lock brake systems, steering wheel components, and air-bag sensors.
- Manufacturing medical laser components, X-ray tube windows, and dental crowns.
- Making nuclear reactor components, missile guidance systems, and heat shields in defense industry.
Safety
Beryllium hydroxide is known to be carcinogenic and repeated exposure through contact, inhalation, or ingestion may cause organ toxicity. It is also hazardous to aquatic life.