Buffer capacity
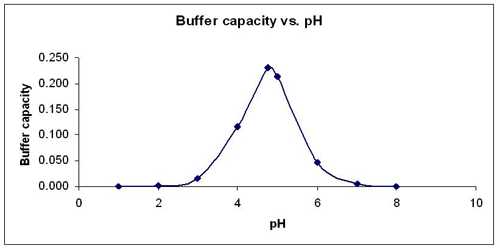
The two most important characteristics of any buffer solution are its pH and buffer capacity. The buffer capacity of any buffer gives a measure of the amount of acid or base that the buffer can react with before changing the pH of the solution significantly. Buffer capacity quantifies the ability of a solution to resist changes in pH by either absorbing or desorbing H+ and OH- ions. When an acid or base is added to a buffer system, the effect on pH change can be large or small, depending on both the initial pH and the capacity of the buffer to resist change in pH.
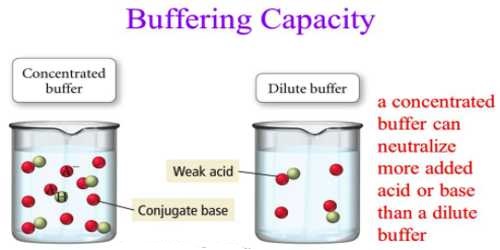
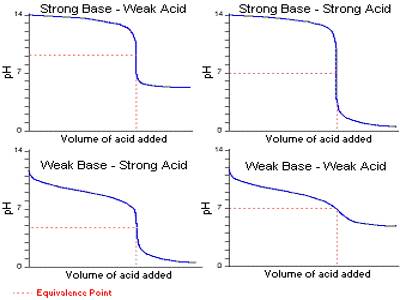
The buffer capacity depends on the amount of acid and its conjugate base (for acid buffer) or the amount of base and its conjugate acid (for basic buffer). The larger is the amounts of acid and its conjugate base or the base and its conjugate acid needed to change the pH of the buffer; the greater is the buffer capacity. Mixtures of solutions of the two components, e.g., salt and acid, which will give buffer of high capacity, can be found from the neutralization curves for the titration of a weak acid with a strong base or a weak base with a strong acid.
In Figure, the region of the curve left of the vertical portion of the graph where there is a mixture of the salt and acid (or base) indicates the buffer mixture. The region where the slope of the curve is very small gives compositions of the mixtures where the buffer capacity is high. In most cases, the buffer capacity is maximum where the concentrations of the salt and acid are equal, i.e. the acid or the base is half neutralized.