Cadmium selenide, abbreviated CdSe, is an inorganic compound. It is a semiconducting material with a direct bandgap in the group II-VI range. It is a black-to-red-black solid classified as an n-type II-VI semiconductor. The nanoparticles of this compound are the focus of much of the current research on it.
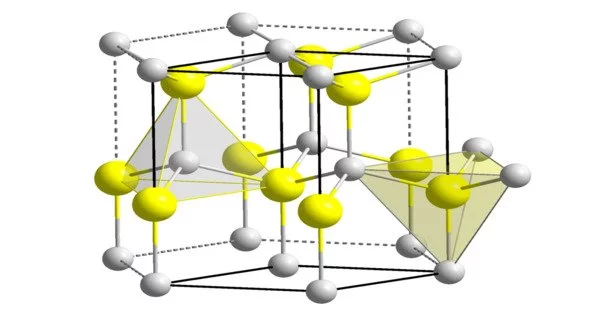
Cadmium selenide is a form of cadmium. It is a tetrahedral semiconductor with a wurtzite structure that has been hexagonally modified. CdSe is an optically uniaxial semiconductor with two dielectric function components.
Properties
Cadmium selenide (CdSe) is a binary compound of cadmium and selenium that is solid. This compound is also known as cadmium (II) selenide, cadmium selenide, and cadmoselite. It is a semiconducting material, but it has yet to find widespread use in manufacturing. This material is transparent to infrared (IR) light and has been used in windows for instruments that use IR light in the past.
- Chemical formula: CdSe
- Molar mass: 191.385 g·mol−1
- Appearance: Black, translucent, adamantine crystals
- Odor: Odorless
- Density: 5.81 g cm−3
- Melting point: 1,240 °C (2,260 °F; 1,510 K)
- Band gap: 1.74 eV, both for hex. and sphalerite
- Crystal structure: Wurtzite
Structure
There are three crystalline forms of CdSe known, which have the following structures: wurtzite (hexagonal), sphalerite (cubic), and rock-salt (cubic). When heated to a moderate temperature, the sphalerite CdSe structure degrades to wurtzite. The transition begins around 130 °C and takes about a day to complete at 700 °C. Only under extreme pressure is the rock-salt structure visible.

Production
The synthesis of cadmium selenide has been accomplished in two ways. The high-pressure Vertical Bridgman method or High-Pressure Vertical Zone Melting is used to creating bulk crystalline CdSe.
Cadmium selenide nanoparticles can also be produced. (Explanation in applications) Several methods for producing CdSe nanoparticles have been developed, including arrested precipitation in solution, synthesis in structured media, high-temperature pyrolysis, sonochemical methods, and radiolytic methods.
The production of cadmium selenide by arrested precipitation in solution is accomplished by introducing alkylcadmium and trioctylphosphine selenide (TOPSe) precursors into a heated solvent under controlled conditions.
Me2Cd + TOPSe → CdSe + (byproducts)
CdSe nanoparticles can be modified by the production of two-phase materials with ZnS coatings. The surfaces can be further modified, e.g. with mercaptoacetic acid, to confer solubility.
Applications
Cadmium selenide is an n-type semiconductor, and its nanoparticles are used in laser diodes, opto-electronic devices, nanosensing, biomedical imaging, and high-efficiency solar cells. Because it is transparent to infrared light, it finds use in photoresistors and windows for instruments that use IR light.
CdSe material is transparent to infrared (IR) light and has seen limited use in photoresistors and windows for instruments that use IR light. The material is also extremely luminescent. CdSe is a component of the pigment cadmium orange.
Natural occurrence
CdSe occurs in nature as the very rare mineral cadmoselite.
Safety information
Cadmium is a toxic heavy metal, and it and its compounds should be handled with extreme caution. Selenides are highly toxic in large quantities. Cadmium selenide is a known carcinogen in humans, so seek medical attention if it is swallowed, inhaled, or comes into contact with skin or eyes.