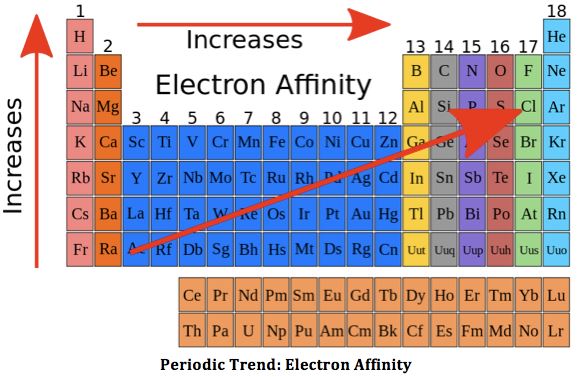
Change of Electron Affinity along a Period: On moving across a period, the size of atoms decreases and nuclear charge increases. Both these factors favour an increase in force of attraction exerted by the nucleus on the electrons.
Consequently, the atom will possess a greater tendency to attract the additional electron, i.e., its electronic affinity would increase as we move from left to right. Due to this reason electron affinities of non-metals are high whereas those of metals are low.
Of all the metals, the E.A. of gold is comparatively high (222.7 kJ mol-1). This value may be attributed to the higher effective nuclear charge and poor shielding of the nucleus by d electrons.












