Nitrogen is a vital nutrient for plant growth. Although about 80 percent of the planet is nitrogen, it is mainly found in the atmosphere as gas and thus unavailable to plants. Ammonia is a primary chemical feedstock for the manufacture of fertilizers and a possible energy carrier. Chemical nitrogen fertilizers are therefore required to boost plant growth, especially in agricultural settings. A key step in the development of these fertilizers is ammonia synthesis, which involves a reaction between hydrogen and nitrogen in the presence of a catalyst.
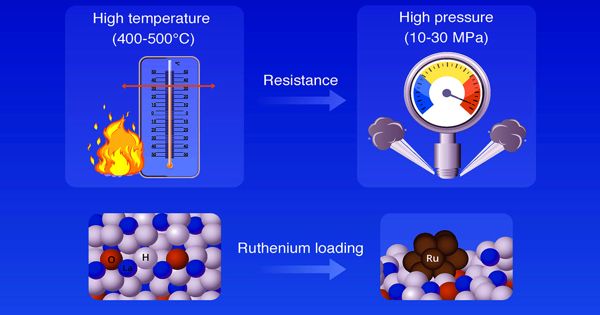
Traditionally, the processing of ammonia has been carried out through the “Haber-Bosch” process, which, while being efficient, involves high-temperature conditions (400-500°C), making the process costly. However, the latest method of synthesizing ammonia, the Haber-Bosch technique, requires a great deal of energy. As a result, scientists have been seeking to find a way to reduce the ammonia synthesis response temperatures. A method and a material that can catalyze ammonia synthesis under moderate conditions (low temperature and low pressure) are strongly required to minimize energy consumption.
Scientists at the Tokyo Institute of Technology (Tokyo Tech) report that the metal ruthenium, supported with lanthanide oxyhydroxides, can efficiently catalyze the synthesis of ammonia at a much lower temperature than the traditional approach. They highlight the advantages of oxyhydroxide support and its potential in becoming a feasible catalyst for low-temperature ammonia synthesis in the future.
Ruthenium is a potential catalyst for ammonia synthesis due to its higher action at low pressure and temperature relative to iron-based catalysts. Recently, scientists have identified ruthenium-a transition metal-as an effective ammonia synthesis “catalyst” because it acts under milder conditions than conventional iron-based catalysts.
Nanostructured catalysts for low-temperature ammonia synthesis have been produced by thermal nitrogen treatment of Ru-containing MOFs. However, there is a caveat: nitrogen molecules need to bind to the surface of the catalyst to be disassociated into atoms before they react with hydrogen to form ammonia. In the case of ruthenium, however, low temperatures also allow hydrogen molecules to bind to its surface instead-a process called hydrogen poisoning-which hinders the production of ammonia. It is also important to suppress its hydrogen poisoning to work with ruthenium.
Fortunately, those materials can improve the catalytic activity of ruthenium when used as a “catalyst support.” A team of scientists from Tokyo Tech, Japan, recently discovered that lanthanide hydride materials of the form LnH2+x are one such category of support materials. “The improved catalytic efficiency is accomplished by two special properties of the supporting material. First, they donate electrons that direct the dissociation of nitrogen on the ruthenium surface. Second, these electrons combine with hydrogen on the surface to form hydride ions, which readily react with nitrogen to form ammonia and release the electrons, suppressing hydrogen poisoning of ruthenium,” explains Associate Prof. Maasaki Kitano, who led the study.
Suspecting that hydride ion mobility might have a role to play in ammonia synthesis, the team, in a new study published in Advanced Energy Materials, investigated the performance of lanthanide oxyhydroxides (LaH3-2xOx) – reportedly fast hydride ion conductors at 100-400°C — as a support material for ruthenium, with the aim of uncovering the relationship between ammonia synthesis and hydride ion mobility.
They observed that while the “bulk” hydride ion conductivity had no effect on the activation of ammonia synthesis, the surface or “local” mobility of hydride ions played a crucial role in catalysis by helping to build up a good resistance to hydrogen ruthenium poisoning. They also observed that lanthanum oxyhydroxides needed a lower onset temperature for ammonia formation (160°C) compared to other support materials and showed better catalytic activity.
In addition, the team indicated that the existence of oxygen stabilized the oxyhydroxide structure and the hydride ions against nitridation—a transformation of lanthanum oxyhydroxide into lanthanum nitride and eventual deactivation—which appears to hinder catalysis and is a major disadvantage in the usage of hydride support materials. “The resistance to nitridation is a tremendous advantage as it helps to preserve the electron-donating ability of the hydride ions for a longer duration of the reaction,” says Prof. Kitano.
The superior catalytic efficiency and lower onset temperature synthesis obtained by using lanthanide oxyhydroxides may therefore be the much sought-after solution to reduce the heat generated by ammonia!