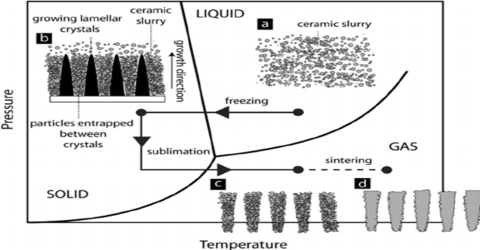
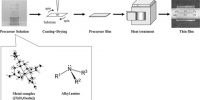
Ceramic method for Preparation of Crystal
Ceramic method: In this method two solids which react to form the required product are heated together either in an ambient condition or in an evacuated vessel. Evacuated vessels are required when the reactant or product is air or water sensitive and volatile.
Solid-State or Ceramic Method consists of heating two non-volatile solids which react to form the required product. The solid-state method can be used to prepare a whole range of materials including mixed metal oxides, sulfides, nitrides, aluminosilicates, etc.
ZrO2 (s) + SiO2 (s) → ZrSiO4 (s) (heat to 1300°C)
The method can be used to prepare an extremely large number of compounds.
The easiest way to make many solid state materials is direct reaction of their components at high temperatures
PbO + TiO2 –(~9000 C)—> PbTiO3
Grind/mix powdered reactants, press into a pellet and heat.
Requires high temperature because reaction is diffusion limited
- can be expensive
- may give incomplete reaction
- may give compositionally inhomogeneous products
Disadvantages
- High temperatures are generally required (500-2000°C), because it takes a significant amount of energy to overcome the lattice energy so a cation or anion can diffuse into a different site.
- The desired compound may decompose at high temperatures.