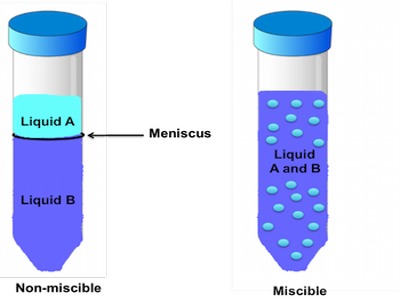
Completely Miscible Liquid Pairs Showing Deviation from Raoult’s Law
Miscibility refers to the ability of a liquid to totally dissolve in another liquid solution. A distinct layer of two liquids will not form when you have a solution that is labeled miscible. When a distinct layer does form in a mixed solution this is called immiscibility. Two types of deviations from Raoult’s Law by non-ideal mixtures are observed:
(A) Positive deviation when the measured partial vapor pressures of the components are more than those expected from Raoult’s law.
(B) Negative deviation if the partial vapor pressures are less than those expected from Raoult’s law.
These deviations are shown schematically in the figure.
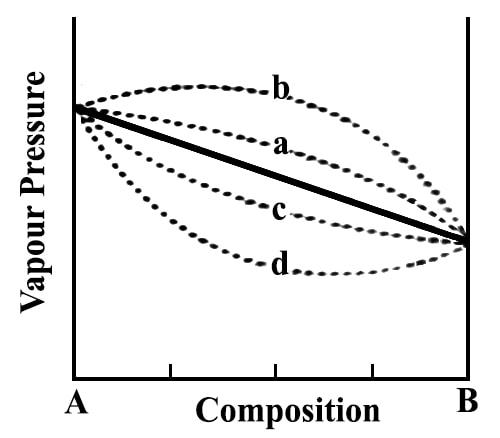
In the case of positive deviation the total vapor pressure as a function of the composition may be shown by the dotted line PA x PB and in the case of negative deviation by the dotted line PA y PB (Figure 11.16). The exact shape of the vapor pressure-composition curves will depend on the type and magnitude of deviation from Raoult’s law. In extreme cases, one may find a maximum or a minimum in such curves.