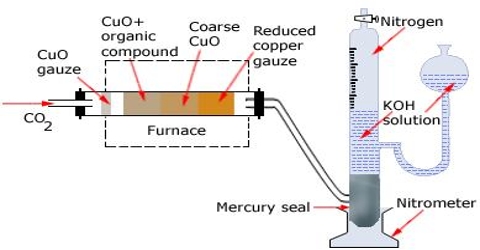
Dumas’ method
It is used for substances which are liquid at room temperature. The apparatus is shown in (Figure: Dumas’ method for determination of density of gas). It consists of a bulb with capacity of about 400 mL and a drawn-out neck. The bulb is first dried and weighed. A few mL of the liquid whose density in the vapour state is to be determined is introduced into the bulb. It is then placed in a bath as shown in the figure so that only the tip of the neck projects out of the liquid in the bath. The bath is then heated to a temperature about 20°C above the boiling point of the liquid in the bulb. The liquid boils, expels air from inside the bulb and then vapour comes out of the neck. When all the liquid is vaporized and the pressure of the vapour within the bulb is, therefore, equal to the atmospheric pressure, the tip is sealed off with a blow torch. The pressure of the atmosphere and the temperature are immediately noted. The bulb is removed, cooled, its outside cleaned and dried, and re-weighed. The difference between this mass and the mass of the bulb when empty gives the mass of the vapour. The volume of the bulb is determined from the density of water and the mass of the water required filling the bulb after breaking the tip of the neck. As the mass and volume of the vapour and the pressure and temperature are known, the molecular mass may be easily calculated.

Only extremely pure substances may be used in Dumas’ method. Since most of the liquid is boiled off, the presence of a small quantity of high boiling impurity may cause a serious error in the results. The method may be used for the determination of densities of vapour of relatively easily volatilized metals (e.g. mercury, zinc etc.) by replacing the glass bulb by bulbs made of porcelain or silica because of the high temperatures required for volatilization.