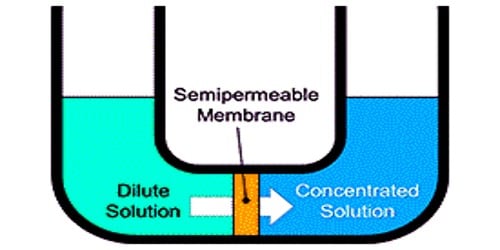
Osmosis is the diffusion of water across a differentially permeable membrane. It is the movement of free water molecules transversely a semi-permeable membrane, down an absorption gradient. It is also the diffusion of water molecules across a semipermeable membrane from a locale of lower absorption solution (for example, a higher concentration of water) to a locale of higher absorption solution (i.e., lower absorption of water).
Free water molecules are the ones that are not interacting with; and thus isolating, solute particles, such a salt ion. Just as with diffusion of other types of molecules, osmosis results in a net dissimilarity in the absorption of water across a membrane, this is a necessary function of biological organisms. (Remember, Salt is too big to pass through a cell membrane, but water molecules can fit). Water moves into and out of cells by osmosis.
The process of osmosis may occur under the following conditions:
- There should have two solutions. One concentrated and other dilute.
- A semi-permeable membrane should separate the two solutions of different concentration.
- The two solutions must be of the same solvent.
- Temperature and atmospheric pressure should be the same.
Osmotic Conditions:
The three types of osmotic conditions that influence living cells are called hypertonic, hypotonic, and isotonic states.
These terms describe the osmotic state of the solution that surrounds a cell, not the solution surrounded by the cell. Hypertonic situation cause water to disperse out of the cell, making the cell shrivels. Hypotonic situations source water to move into the cell swelling or bursting it. Lastly, an isotonic situation also allocates the movement of water in and out of the cell, but with no net augment inside or outside of the cell.
Osmotic Pressure (OP):
In the process of osmosis pressure required from higher concentration area to stop the movement of solvent from lower concentrated solution to higher concentrated solution when a semi-permeable membrane separates the two solutions, is called Osmotic Pressure (OP). Osmotic pressure is defined as the hydrostatic pressure required to stop the net course of water transversely a membrane extrication solutions of dissimilar compositions. More the difference of concentration of two solutions more the OP, and less the difference of concentration, less will be the OP. if the concentration of two solutions is the same there will have no OP and osmosis will not run. In this situation, the “membrane” might be a layer of cells or a plasma membrane.
Turgor Pressure (TP):
Due to endosmosis water enter into the cell and the cell becomes swollen. This swollen condition of the cell is called turbidity. Due to turgidity, the protoplasm exerts a pressure on the cell wall; this pressure is called Turgor Pressure. Turgor Pressure protoplasts of healthy plant cells are immersed in a hypotonic medium. In this circumstance, water tends to move into the protoplast due to osmosis.
However, the protoplast is surrounded by a cell wall which limits its volume. This circumstance generates pressure enough to stop the net movement of water. This pressure is termed ‘turgor pressure’. Turgot pressure is significant in the sustain of herbaceous plant tissues.