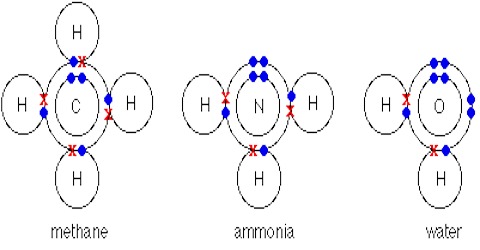
During chemical interaction, of two atoms of the same element or different elements, if transfer of outer-shell electrons is not possible, then by sharing of electrons in pairs between the atoms a stable electronic bond is formed in the outer shell. This type of bond is called covalent bond.
So, during the formation of molecule, if atoms in order to form stable inert gas electronic configuration and for that purpose if the atoms share electrons between their outer shell then that bored is the covalent bond. This bond is also known as valence bond. Due to sharing of electrons density of electron in the middle region becomes higher. This type of bonding is clearly directional. Covalent bond is a strong bond.
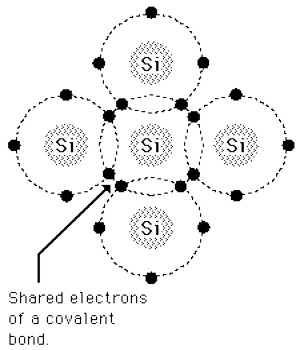
Example: Hydrogen, nitrogen, silicon. In figure covalent bond of Si is shown.
Conditions for covalent bond:
(i) Covalent bond occurs between two non-metal atoms.
(ii) By providing one or more electrons between two non-metallic atoms an electron couple is created which is shared by each of the atoms equally.