Reversible process:
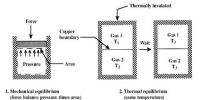
A thermodynamic process in which any sort of change of a working substance in the external condition, starting from a given initial can he brought back in the opposite direction so that the working substance passes through exactly the same state in all respect is known as the reversible process.
The working substance in Carnot’s engine during its course of operation gets back to its initial state, so this type of thermal change is reversible.
The changing of water to ice or ice to water and water to vapour or vapor to water is also the example of the reversible process.
Irreversible Process:
The thermodynamic process in which the working substance does not regain its original state after it changes thermodynamically is called irreversible Process.
In this process some energy loss to overcome friction and dissipating a portion by conduction and radiation. This type of energy cannot regain the system during its reverse and the system cannot reach its initial state.
When current is passed through a copper wire, the light, as well as heat, is produced. In this case, light and heat can never he turn to electricity or current.