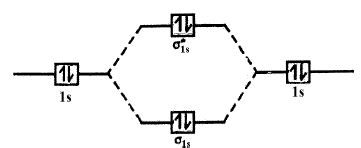
Diatomic helium molecule (He2 hypothetical) is the electronic configuration of helium (Z = 2) in the ground state is 1s2. As each helium atom contains two electrons, there will be four electrons in He2 molecule. Keeping in view the Aufbau principle and Pauli’s exclusion principle its electronic configuration would be as follows.
He2: (σ1s)2 (σ*1s)2
The molecular orbital energy level diagram of He2 (hypothetical) is given in Figure.