A team of organic chemists from the Max-Planck Institute for Solid-State Research, in collaboration with colleagues from the Universities of Tübingen and Copenhagen, has developed a method for photographing the sequences and locations of glycans (also known as polysaccharides) bound to several biomolecules at the single-molecule level. Their findings were reported in the journal Science.
Glycans are carbohydrates that are involved in a variety of biological activities, including protein folding. They are commonly present on the outsides of most cells. Previous study has shown that they can be branched or linear and are composed of O-glycosidic connections of monosaccharides.
Scientists have been researching them for many years due to their importance in both biological processes and research activities. In this new endeavor, the research team created a microscope method for photographing glycans as they bond to proteins.
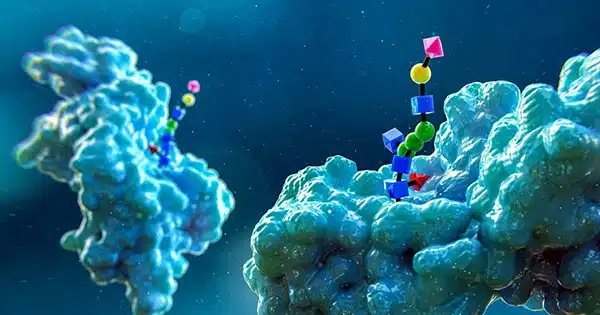
The scientists discovered an electrospray approach that pushed glycans linked to lipid and protein molecules (known as glycosaminoglycans and glycoconjugates) onto two metal surfaces—silver and copper—after conducting a slew of experiments seeking a means to photograph glycan binding. They were able to view the molecules directly using scanning tunneling microscopy as a result of this.
The researchers were able to identify specific monosaccharides in a glycan chain, allowing them to understand more about how glycans are orientated and where they connect to protein backbones.
The researchers also showed their new imaging technique by photographing oxygen-linked glycans while they were coupled to mucin proteins—images that, they say, could be beneficial in the search for early cancer biomarkers. The technique could be applied to a wide range of research projects, including the discovery of previously undiscovered glycolipids and/or glycoproteins.