Distillation of Non-ideal Solutions: Azeotropic Mixture
An azeotrope is a mixture that exhibits the same concentration in the vapor phase and the liquid phase. Liquid pairs that show large deviations from Raoult’s law may have either a maximum or a minimum in the vapor-pressure curve, as shown in Figure 1, depending on whether the deviation is positive or negative. A maximum in the vapor pressure curve leads to a minimum in the boiling point curve; while a maximum in the boiling point curve corresponds to a minimum in the vapor pressure curve. If the mixture forms an azeotrope, the vapor and the liquid concentrations are the same, preventing separation via this approach. As will be discussed shortly, in such cases separation of the liquid pairs by fractional distillation alone is not possible.
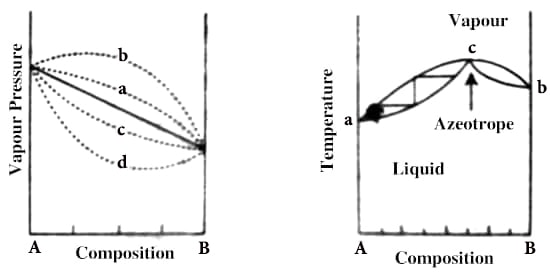
Fig: 1: p-c diagram of systems showing deviation from Raoult’s law
Fig: 2: t-c diagram of systems shown
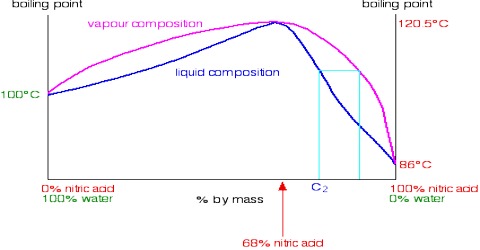
The t – c curve of a mixture showing a maximum is shown in Figure 2, the upper curve is for the vapor while the lower one is for the liquid as in previous cases, and a and b are the boiling points of the pure components. At point c, the mixture has a maximum boiling point and the liquid and vapor in equilibrium have the same composition. If a mixture of composition c is vaporized and then condensed the distillate will have the same composition as the original liquid. A mixture that distills without a change in composition is known as an azeotrope or an azeotropic mixture. A common example of such systems is H2O-HCl.
Azeotropes are a mixture of at least two different liquids. Their mixture can either have a higher boiling point than either of the components or they can have a lower boiling point. When a mixture forms an azeotrope, complete separation of the liquids by simple fractional distillation is not possible. Referring to Figure 2, if one starts with a mixture having a composition corresponding to a point to the left of the azeotrope, the distillate will gradually be enriched in A but the liquid will become richer in B until the azeotropic composition is reached. If, on the other hand, one starts with the liquid mixture of composition corresponding to a point to the right of the azeotrope the liquid will get enriched in A until the azeotropic composition is reached. The liquid of this composition distills unchanged in composition.
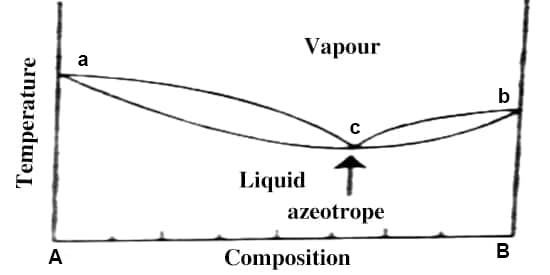
Fig: t-c diagram
The situation becomes similar if the t-c diagram has a minimum as shown in Figure 3. From whatever side of the azeotrope one may start distillation, the composition soon reaches the azeotropic value and the solution continues to boil at a constant temperature like a pure liquid and prevents any further separation. Alcohol-water, n-propanol-water acetone-carbon disulfides are a few examples of liquid pairs showing minimum in the t-c diagram.
Azeotropes occur when a fraction of the liquids cannot be altered by distillation. Typically when dealing with mixtures, components can be extracted out of solutions by means of Fractional Distillation, or essentially repeated distillation in stages. The azeotropic mixtures boil at constant temperatures and distill the user without any change of composition at a given pressure. If, however, the pressure is changed, both the boiling temperatures as well as composition change. This is unlike pure compounds. For pure compounds the boiling temperature as changed with changes for pressure, but not the composition. For example, H2O boils at 1000C at atmospheric pressure but on the top of a mountain, the boiling temperature will be much lower because the pressure is lower. The composition, however, will always be H2O. In other words, an azeotropic is a mixture and not a compound.