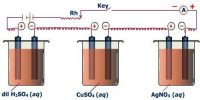
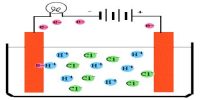
Electrolysis is the process of chemical decomposition of an electrolyte either in the fused state or in an aqueous solution by the passage of electric current through it. During electrolysis, positive ions migrate to the cathode and negative ions migrate to the anode. They are liberated at the respective electrodes either by accepting or by losing electrons. Hence, electrolysis is an oxidation-reduction process. During electrolysis, cation receives one or more electron from cathode i.e. it is reduced. On the other hand, anion donates one or many electrons to anode i.e. it is oxidized. For example, during the electrolysis of NaCI, the following processes occur.
NaCI —> Ne+ (I) + Cl– (I)
Reduction at cathodes:
Na+ + e– —> Na
Oxidation at the anode:
Cl– —> Cl + e–
In reality, if we observe electrolysis we will observe that reduction in cathode and oxidation in anode takes place. So, electrolysis is an oxidation-reduction process.