Enthalpy diagrams are a representation of the relative internal energy of a system before and after a reaction. Horizontal lines are drawn to show the energy of the system at a particular time. The nature of the system which has that energy is written on the line. The formulae of all the elements or compounds present in the system including their state: solid, liquid or gas. There is no scale on the axis, as absolute enthalpy cannot be measured. A line at a higher level merely shows that the enthalpy of the system has increased due to the addition of heat from the surroundings, which has caused a physical change (e.g. melting) or chemical change (i.e. a reaction) in the system. The x-axis can be labelled progress of the reaction.
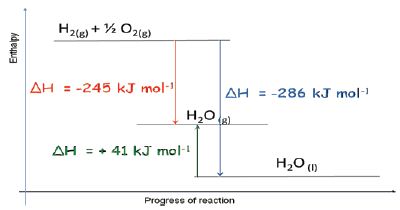
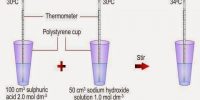
Enthalpy and Enthalpy Change There is no way of measuring the enthalpy of any single substance directly. For this reason, we can only discuss enthalpy changes in a reaction and this can be measured as an amount of heat given out (exothermic reaction) or taken in (endothermic reaction).
Example
When concrete is mixed, the main reaction is between calcium oxide and water CaO (s) + H2O (I) 4 Ca(OH)2 (s) ΔH = -65.2 kJ mol-1
This reaction gives out a large amount of heat – so much that when building a large concrete structure such as a dam, cooling pipes must be included to carry away the heat.