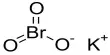Individuals with Type 1 diabetes must carefully adhere to daily insulin regimens, getting injections of the hormone via syringe, insulin pump, or other devices. And, in the absence of viable long-term cures, this course of treatment constitutes a life sentence. A group of researchers has now identified a better method.
When blood sugar levels alter, pancreatic islets govern insulin production, and in Type 1 diabetes, the body’s immune system targets and destroys such insulin-producing cells. Islet transplantation has emerged as a potential therapy for Type 1 diabetes in recent decades. Type 1 diabetes patients may no longer require insulin injections if they receive healthy transplanted islets, however, transplantation attempts have been hampered since the immune system continues to reject new islets. Immunosuppressive medications now available provide insufficient protection for transplanted cells and tissues and are fraught with unfavorable side effects.
Now, a team of Northwestern University researchers has developed a method to improve the efficacy of immunomodulation. The approach reengineers the commonly used immunosuppressant rapamycin using nanocarriers. The researchers created a new type of immunosuppression using these rapamycin-loaded nanocarriers, capable of targeting specific cells relevant to the transplant while suppressing broader immune responses.
The findings were published in the journal Nature Nanotechnology.
This approach can be used to other transplanted tissues and organs, opening up new research avenues and treatment alternatives for patients. We are now working to bring these very promising findings one step closer to clinical application.
Professor Dr. Ameer
Specifying the body’s attack
Ameer has been attempting to improve the outcomes of islet transplantation by giving the islets with an artificial habitat that uses biomaterials to optimize their life and function. However, difficulties associated with traditional systemic immunosuppression continue to be a barrier to clinical treatment of patients and must be addressed in order to effectively improve their care, according to Ameer.
“This was an opportunity to collaborate with Evan Scott, a leader in immunoengineering, on a convergent research project that was skillfully handled with excellent attention to detail by Jacqueline Burke, a National Science Foundation Graduate Research Fellow,” Ameer explained.
Rapamycin is well-studied and widely used to suppress immune responses during different types of treatment and transplants, and it is notable for its broad effects on numerous cell types throughout the body. Rapamycin is often administered orally, and its dosage must be carefully controlled to avoid harmful consequences. However, at lesser doses, it is ineffective in conditions such as islet transplantation.
Scott, a CARE member, said he wanted to investigate how the medicine could be improved by encapsulating it in a nanoparticle and “managing where it goes within the body.”
“To minimize the wide effects of rapamycin throughout treatment,” Scott explained, “the medicine is normally administered at modest doses and via specialized routes of administration, primarily orally.” “However, in the case of a transplant, you must administer enough rapamycin to systemically inhibit T cells, which can result in major adverse effects such as hair loss, mouth ulcers, and a weakened immune system.”
T cells, which are immune cells, will reject freshly introduced foreign cells and tissues after a transplant. Immunosuppressants are used to counteract this effect, but they can also impair the body’s ability to fight other infections by suppressing T cells across the body. However, the team designed the nanocarrier and medication combo to have a more targeted effect. Rather than directly influencing T cells, which is the most common therapeutic target of rapamycin, the nanoparticle would be designed to target and change antigen presentation cells (APCs), allowing for more targeted, controlled immunosuppression.
The team was also able to give rapamycin by subcutaneous injection, which they discovered employs a distinct metabolic pathway to minimize substantial drug loss in the liver after oral treatment. To be effective, this route of delivery requires substantially less rapamycin – roughly half the normal dose.
“We wondered if rapamycin could be re-engineered to prevent non-specific inhibition of T cells and instead boost a tolerogenic pathway by targeting distinct types of immune cells?” Scott stated. “We modified the method immunosuppression was achieved by modifying the cell types that were targeted.”
A ‘pipe dream’ come true in diabetes research
The researchers tested their idea on mice by instilling diabetes in the population before administering a combination of islet transplantation and rapamycin via the usual Rapamune® oral regimen and their nanocarrier formulation. Mice were given injections of the modified medication starting the day before transplantation and every three days for two weeks.
The scientists saw few adverse effects in the mice and discovered that the diabetes was eradicated for the duration of their 100-day experiment; nevertheless, the treatment should last the duration of the transplant. The researchers also found that animals treated with the nano-delivered medicine showed a “robust immunological response” when compared to mice given normal pharmacological treatments.
According to Scott, the concept of increasing and managing pharmacological adverse effects by nanodelivery is not novel. “But in this case, we’re not boosting an impact, we’re changing it — by repurposing a drug’s metabolic pathway, in this case mTOR inhibition by rapamycin, we’re generating a whole different cellular response.”
The discoveries made by the scientists could have far-reaching consequences. “This approach can be used to other transplanted tissues and organs, opening up new research avenues and treatment alternatives for patients,” Ameer added. “We are now working to bring these very promising findings one step closer to clinical application.”
Jacqueline Burke, the study’s lead author and a National Science Foundation Graduate Research Fellow and researcher at CARE who collaborated with Scott and Ameer, said she couldn’t believe her eyes when she watched the mice’s blood sugar drop from very diabetic levels to an even amount. She double-checked to make sure it wasn’t a fluke, but the number remained consistent over the course of months.
Research hits close to home
Burke, a doctorate student studying biomedical engineering, finds the research more personal. Burke is one such person, and daily shots are a well-known aspect of her life. She was diagnosed with Type 1 diabetes when she was nine years old and knew she wanted to make a difference in the field for a long time.
“At my last program, I worked on wound healing for diabetic foot ulcers, a consequence of Type 1 diabetes,” Burke explained. “As a 26-year-old, I never want to get there, so I thought it would be a better strategy to focus on how we can manage diabetes now in a more succinct way that matches the natural happenings of the pancreas in a non-diabetic individual.”
The all-Northwestern research team has been doing tests and publishing articles on islet transplantation for three years, and Burke and Scott both agree that the work they just published could have been split into two or three pieces. What they’ve published now, however, they consider a breakthrough and suggest it could have far-reaching ramifications for diabetes research in the future.
Scott has started the process of patenting the approach and cooperating with industrial partners in order to eventually bring it into clinical testing. Commercialization of his work would overcome the remaining challenges for emerging technologies such as Vertex’s stem-cell produced pancreatic islets for diabetes treatment.