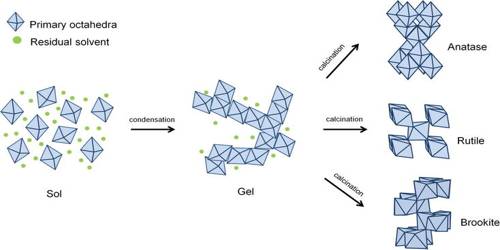
All Gels are basically Sols but all Sols are not Gels
If the sol is concentrated and the discrete particles are present in a state of bridged or cross-linked structure the system is called a gel. A gel is mechanically stable and possesses elasticity. Ordinary jelly or jello is an example of gel. All gels are basically cols but all sols are not necessarily gels. There are cases in which sol-gel transformation and vice versa are possible. The main distinction between sol and gellies in the pseudo-regular structure of particles in a gel whereas in a sol the-particles are wide apart in a completely irregular fashion.
Moreover, in a gel the liquid entrapped within the bridged or cross-linked structure is immobilized, i.e., this liquid is not available for free flow but moves along with the whole gel as if it is an integral part of the whole structure. This is shown in Figure.
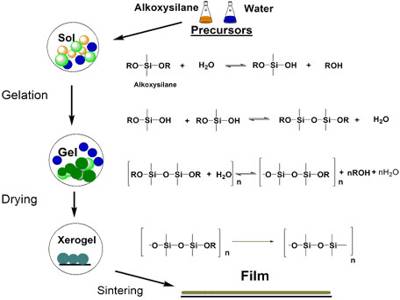
Fig: Schematic representation of a sol-gel structure.
Many gels, simply by vigorous agitation, can become fluid again, giving a sol, but on standing reverts back to gel structure. For example, if a concentrated ferric hydroxide sol is allowed to stand undisturbed in a test tube it will set to a gel. The gel will not fall even when the test tube is inverted; if it falls at all, the whole gel will fall like a plug. But if the test tube is shaken vigorously and then inverted, the mass will undergo flow indicating that gel structure is broken and sol has been formed.