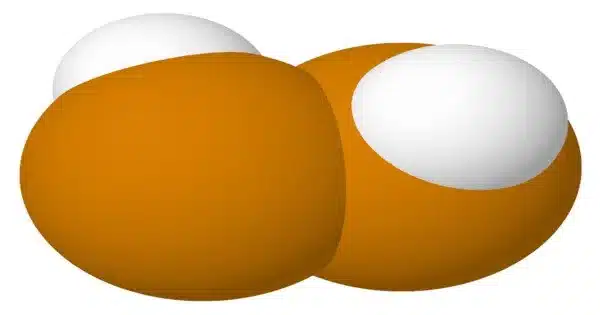
Hydrogen ditelluride, also known as ditellane, is an unstable hydrogen dichalcogenide with the structure HTeTeH or (TeH)2. Theorists are interested in hydrogen ditelluride because its molecule is simple yet asymmetric (it lacks a center of symmetry) and is predicted to be one of the easiest to detect parity violations, in which the left-handed molecule differs from the right-handed one due to the effects of the weak force.
Tellurium is a rare element with a valence of -2, which means it prefers to gain two electrons in order to achieve a stable electron configuration. Tellurium would need to gain four electrons to form Te2- ions in the case of hydrogen ditelluride. As a result, the compound would be highly unstable and reactive.
Properties
- Physical State: Hydrogen ditelluride is a gas at room temperature and atmospheric pressure. It has a boiling point of approximately -2.2 degrees Celsius (-36 degrees Fahrenheit) and a melting point of -49.2 degrees Celsius (-56.6 degrees Fahrenheit).
- Odor: Hydrogen ditelluride has a characteristic foul odor, often described as a cross between rotten eggs and garlic. The odor is noticeable even at very low concentrations.
- Solubility: It is sparingly soluble in water, meaning it dissolves only to a limited extent. The solubility increases with decreasing temperature.
- Density: The gas has a density of approximately 5.7 grams per liter at standard temperature and pressure (STP).
- Stability: Hydrogen ditelluride is a highly unstable compound. It decomposes easily, especially upon exposure to heat or light, releasing toxic fumes of tellurium. It can also react violently with oxidizing agents.
Production
In acid electrolysis, hydrogen ditelluride may be formed at the tellurium cathode. When a tellurium cathode is electrolyzed in alkaline solutions, it produces ditelluride Te2-2 ions as well as Te2- and a red polytelluride. When the pH exceeds 12, the most ditelluride is produced. Ditellane has been detected in the gas phase produced from di-sec-butylditellane, in addition to its speculative detection in electrolysis.
Furthermore, due to its instability, hydrogen ditelluride is not well studied or commonly encountered. The compound is not commercially available, and its properties and potential applications, if any, are unknown. When dealing with uncharacterized compounds, it is critical to exercise caution because they may pose significant risks or hazards.
Toxicity
Hydrogen ditelluride is extremely toxic and dangerous to one’s health. Inhaling or being exposed to the gas can cause severe respiratory irritation, eye irritation, and central nervous system damage. It is critical to use proper safety precautions when handling the compound.