Importance of Osmosis Phenomenon
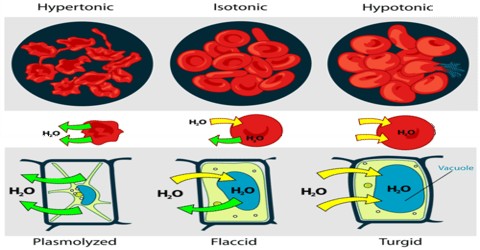
Osmosis is an important phenomenon in many biological and technological processes. Animal and vegetable cells containing solutions of salts and sugars (cell saps) are surrounded by semi-permeable membranes. The osmotic pressure of the solution surrounding the cell must be equal to that within the cell. If such a cell is placed in water or solution having lower osmotic pressure than that of the cell sap, water enters into the cell causing haemolysis. On the other hand, if such a cell is immersed in a solution of higher osmotic pressure, water will pass out through the cell and consequently lead to plasmolysis. The flow of water from the soil to plants is due to osmosis. The osmotic pressure inside the plant cell in the root in contact with soil is higher than that inside the plant cell. As a result water from soil flows into the cell by osmosis. Plant movement such as opening and closing of flowers and leaves are also regulated by osmosis.
As has already been discussed, in osmosis solvent molecules flow through a semi-permeable membrane from pure wheat so the solution, or from a dilute solution to concentrated solution. We also know that the flow of solvent, (i.e., osmosis) through the semi-permeable membrane can be stopped by applying equal pressure to the solution side. If, however, the applied pressure is higher than the osmotic pressure, osmosis is forced to take place in the opposite direction, i.e., from solution to solvent. This is called ‘reverse osmosis’. This concept has been applied in many countries to remove salinity ocean water.