Cancer is the world’s second leading cause of death. There were 18.1 million new cancer cases and 9.5 million cancer-related deaths worldwide in 2018. By 2040, the number of new cancer cases per year is expected to reach 29.5 million, with 16.4 million cancer-related deaths.
Approximately half of all cancer patients can benefit from radiotherapy in their disease management. Approximately half of those patients are diagnosed at an early enough stage that their cancer may be curable. Curative treatment for many cancers, including breast, prostate, cervix, head and neck, lung, and brain cancers, includes radiation therapy. However, because radiotherapy destroys healthy cells as well as tumor cells, doses are limited.
Radiotherapy, also known as radiation therapy, is used to treat cancer alone or in conjunction with other treatment options such as chemotherapy and surgery. It may also be used to reduce the size of the tumor prior to surgery. Tumor cells, which divide much faster than surrounding healthy cells, are destroyed by radiotherapy by damaging their DNA.
Radiation therapy is used alone to treat cancer or with other treatment options such as chemotherapy and surgery. It may also be used to shrink the tumor before surgery.
Side-effects limit radiation dose
The limiting factor in radiotherapy is that high doses used to try to cure high-risk (locally advanced) non-metastatic tumors also cause damage to surrounding normal tissues. Currently, we have reached the upper limit of radiotherapy doses that can be administered to patients. To improve survival even further, new methods that increase radiation effectiveness while reducing side effects are required.
One method is to make tumor cells more sensitive to radiation so that they are more easily damaged by radiation therapy. The use of gold nanoparticles as radiosensitizers has yielded encouraging results. These gold nanoparticles can be introduced intravenously and accumulate in the tumor by taking advantage of the faulty walls of the tumor’s blood vessels, which are leaky due to the tumor’s rapid growth.
Gold nanoparticles interact with X-ray photons used in radiation therapy, producing electrons that then interact with water molecules, producing free radicals. These free radicals can cause cell damage, reducing cell survival.
Understanding the complex biological system present in and around the tumor is essential for optimizing the use of the radiosensitizing GNPs, as outlined by a consortium of labs, including our own nanoscience and technology development laboratory at the University of Victoria.
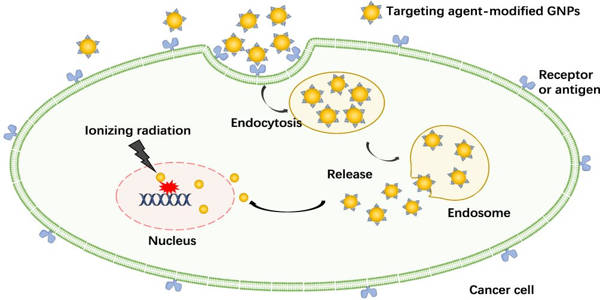
Targeting interactions inside the tumor
In this paper, we discuss how important it is to investigate which cellular components in the tumor microenvironment absorb gold nanoparticles and become radiosensitizers. We are particularly interested in activated fibroblasts, which are associated with wound healing and have anti-tumorogenic properties, which means they aid in tumor growth.
Activated fibroblasts, on the other hand, can be recruited by tumor cells and become cancer-associated fibroblasts (CAFs). CAFs, rather than having anti-tumourigenic properties, promote tumor proliferation and metastasis.
CAF function supports the notion that tumors are “wounds that do not heal,” and targeting CAFs may help improve cancer treatment outcomes.
To be effective in improving radiation treatment, the cells targeted by the treatment (those associated with cancer growth) must have a high uptake of radiosensitizing particles, while the beneficial cells must have low uptake. This allows radiation therapy to more easily destroy the targeted cells at doses that patients can tolerate.
These findings using 3D tumors grown in the lab are very promising. CAFs had nearly triple the number of gold nanoparticles per cell as cancer cells, while fibroblasts had a relatively low number. This resulted in a greater increase in DNA damage in CAFs compared to other cell types, reducing CAF activity and slowing tumor growth.
This difference in DNA damage caused by the selective targeting of cancer-associated cells over normal cells may allow gold nanoparticles to be an effective tool in future cancer radiation therapy, reducing damage to normal tissue while increasing local radiation therapy dose to the tumor.
This study shows that using gold nanoparticles as a radiosensitizer allows more damage to be propagated to the CAFs, which has been shown to be a major factor in cancer progression. We believe that this research will serve as a foundation for a more effective treatment regime in the near future. Building a model that accurately represents the various interactions occurring within the tumor’s microenvironment is critical to improving patient treatment outcomes.