A molecule will have an overall dipole (i.e. be polar) if: It has polar bonds and The overall shape of the molecules is such that the individual dipoles do not cancel.
Attractive forces between separate molecules (sometimes known as Van der Waals forces). For polar molecules, attractive forces are:
- Dipole-dipole attractions
- Hydrogen bonding (H-bonding)
- London dispersion forces (very weak)
For non-polar molecules, attractive forces are London dispersion forces only
The greater the attractive forces between molecules, the more difficult it is to separate them and the higher the melting and boiling points. Intermolecular forces are much weaker than the covalent bonds inside the individual molecules (intramolecular).
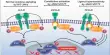
Dipole-dipole attractions: Any molecule with a dipole will be attracted to other polar molecules.

The δ+ end of each molecule will be attracted to the 8- end of a neighbouring molecule.