Fluorine does not occur free in nature. It occurs in the combined form. This was devised by Dennis, Veeder and Rochow in 1931.
- Symbol – F; Atomic number -9; Period Number: 2
- Valency –1 Atomic mass–19 Group Number: 17
In this fluorine is prepared by the electrolysis of fused sodium or potassium hydrogen fluoride (perfectly dry) Electrolysis is carried out between graphite electrodes in a V-shaped electrically heated copper tube. The ends of the tube are covered with copper caps into which the graphite electrodes are fixed with bakelite cement. The copper tube is thickly lagged to prevent loss of heat.
KHF2 → KF + HF
HF → H+ + F¯
2H+ + 2e– → H2 (At cathode)
2F– – 2e– → F2 (At anode)
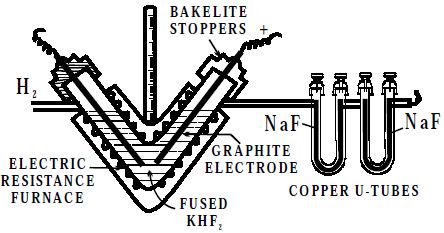
Fig: Preparation of fluorine
Fluorine liberated at the anode is passed through the U-tube containing sodium fluoride. This removes the hydrogen fluoride vapours coming with fluorine.
NaF +HF →NaHF2
Physical Properties
- Fluorine is a gas and has pale greenish yellow colour.
- It has extremely pungent and penetrating odour.
- It is heavier than air.
Chemical Properties
Fluorine is the most active member of halogen family.
- Action with Hydrogen: Hydrogen explodes violently in fluorine even in the dark.
H2 + F2 → 2HF
- Action with non-metals: Non-metals like carbon, silicon and phosphorus burn in fluorine forming fluorides.
C + 2F2 → CF4 (Tetra fluoromethane)
Si + 2F2→ SiF4 (Silicon tetrafluoride)
2P + 5F2 → 2PF5 (Phosphorus pentafluoride)
Action with metals: It reacts with metals forming corresponding fluorides.
- 2Ag + F2→ 2AgF
- 2Al + 3F2→ 2AlF3