Phosphorus Acid (H3PO3) is prepared by the action of cold water on phosphorus (III) oxide or phosphorus (III) chloride.
- P2 O3 + 3H2O → 2H3PO3
- PCl3 + 3H2O → H3PO3 + 3HCl
Physical properties
It is a white crystalline solid with garlic taste.
Chemical Properties
1. Acidic nature: It is a dibasic acid and gives salts of two types.
- H3PO3 + NaOH → NaH2PO3 (Sodium dihydrogen Phosphite) + H2O
- H3PO3 + 2NaOH → Na2HPO3 (Disodium hydrogen Phosphite) + 2H2O
2. When it is heated it undergoes auto-oxidation and reduction to form phosphoric acid and phosphine.
- 4H3PO3 → 3H3PO4 + PH3
3. It is a powerful reducing agent because it has P-H bond. It reduces silver nitrate solution into silver.
- 2AgNO3 + H3PO3 + H2O → 2Ag +H3PO4 +2HNO3
Electronic structure
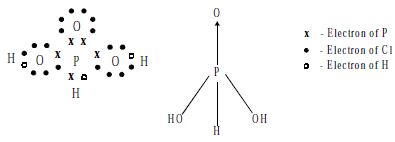 Use: It is used as a reducing agent.
Use: It is used as a reducing agent.