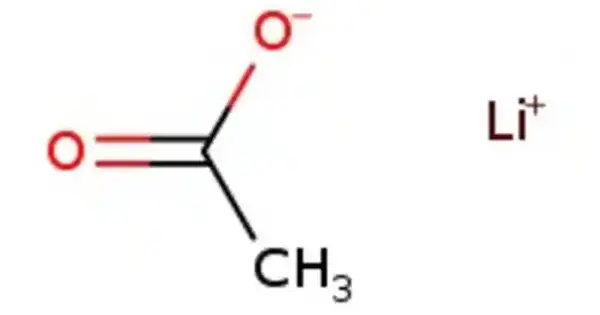
Lithium acetate (CH3COOLi) is a lithium and acetic acid salt. It is frequently abbreviated as LiOAc. It is the acetic acid lithium salt. The chemical is frequently employed in molecular biology and biochemistry, particularly in yeast transformation methods. Water and some organic solvents dissolve it.
Lithium acetate is extensively employed in molecular biology as a carrier in the precipitation of DNA or RNA, assisting in the isolation and purification of nucleic acids. It is also utilized in yeast cell transformation, where it aids in the introduction of foreign DNA into the cells. Lithium has been explored for its possible therapeutic benefits, particularly in the treatment of bipolar illness, in addition to its involvement in molecular biology. However, the use of lithium compounds in medicine is different from the use of lithium acetate in laboratory procedures.
Properties
- Chemical formula: C2H3LiO2
- Molar mass: 65.98 g·mol−1
- Appearance: crystal
- Density: 1.26 g/cm3
- Melting point: 286 °C (547 °F; 559 K)
- Solubility in water: 45.0 g/100 mL
- Magnetic susceptibility (χ): −34.0·10−6 cm3/mol
Uses
In the laboratory, lithium acetate is employed as a buffer for gel electrophoresis of DNA and RNA. It has lower electrical conductivity and can be run at faster speeds than TAE buffer gels (5-30V/cm vs. 5-10V/cm). Because the heat generation and hence the gel temperature are substantially lower at a given voltage than with TAE buffers, the voltage can be adjusted to speed up electrophoresis such that a gel run takes only a fraction of the typical time. When employing lithium acetate gels, downstream applications such as DNA separation from a gel slice or Southern blot analysis perform as predicted.
When examining smaller fragments of DNA (less than 500 bp), lithium boric acid or sodium boric acid are frequently preferred over lithium acetate or TAE due to the better resolution of borate-based buffers in this size range compared to acetate buffers.
Lithium acetate is also used to permeabilize yeast cell walls for DNA transformation. The positive impact of LiOAc is thought to be due to its chaotropic activity, which denaturates DNA, RNA, and proteins.