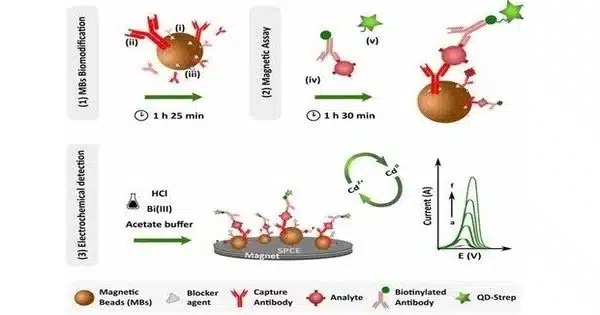
Magnetic Immunoassay (MIA) is a form of diagnostic immunoassay that uses magnetic beads as labels instead of conventional enzymes (ELISA), radioisotopes (RIA), or fluorescent moieties (fluorescent immunoassays) to detect a specific analyte. It is a diagnostic approach that combines immunology and magnetism principles to identify and measure certain molecules, usually biological markers such as proteins, antibodies, or antigens.
MIA is the specific binding of an antibody to its antigen in which a magnetic label is attached to one of the pair’s elements. A magnetic reader (magnetometer) detects the presence of magnetic beads and measures the magnetic field shift caused by the beads. The signal measured by the magnetometer is proportional to the analyte (virus, toxin, bacteria, cardiac marker, etc.) concentration in the initial sample. This technique is commonly used in medical diagnostics, research, and various other fields.
How a magnetic immunoassay generally works:
- Antibody-Antigen Interaction: The specific binding of antibodies to antigens is the foundation of any immunoassay. Specific antibodies are immobilized onto a solid substrate, such as magnetic beads or a surface like a microtiter plate, in a magnetic immunoassay. These antibodies are programmed to bind only to the target molecule (antigen).
- Sample Introduction: The system is fed a sample containing the antigen of interest. If the antigen is present in the sample, it will attach to the immobilized antibodies, resulting in the formation of antibody-antigen complexes.
- Washing: After giving enough time for binding, the unbound molecules are removed by a washing procedure. This step guarantees that only the relevant complexes stay linked to the solid support.
- Magnetic Separation: This is the crucial step that distinguishes magnetic immunoassays from ordinary immunoassays. Magnetic beads are frequently utilized as a firm support. Typically, these beads are coated with a magnetic substance, such as iron oxide nanoparticles. When a magnetic field is applied to the beads, the beads and the linked antibody-antigen complexes are attracted to the magnet, allowing the complexes to be easily separated from the remainder of the sample.
- Detection: Once the magnetic beads containing the bound complexes have been separated, several methods for detecting the presence and quantity of the target antigen can be applied. Fluorescence, chemiluminescence, and enzyme-linked reactions are common detection methods. The magnitude of the produced signal is proportional to the concentration of the target antigen in the sample.
Several advantages
- Enhanced Sensitivity: The use of magnetic separation allows for improved sensitivity and reduced background noise, leading to more accurate results.
- Faster Assays: Magnetic separation is a rapid process, reducing the overall assay time.
- Automation: Magnetic immunoassays are well-suited for automation, making them suitable for high-throughput applications.
- Multiplexing: Multiple target molecules can be detected simultaneously by using different types of magnetic beads, each coated with specific antibodies.
- Reduced Sample Volume: Magnetic immunoassays often require smaller sample volumes than traditional immunoassays.