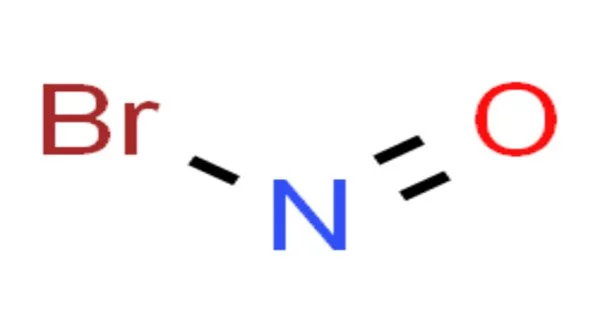
Nitrosyl bromide is a chemical compound having the formula NOBr. It’s a red gas with a condensing point just below room temperature. It is a covalent molecule with one nitrogen atom, one oxygen atom, and one bromine atom bound together. It is not usually encountered in practical applications due to its volatility.
Preparation
Nitrosyl bromide can be made by reacting bromine (Br2) with sodium nitrite (NaNO2) in the presence of an acid. It can be produced via the reversible reaction of nitric oxide with bromine. This reaction is interesting since it is one of the few third-order homogeneous gas reactions. At standard pressure and temperature, NOBr is photodissociable.
2 NO + Br2 ⇌ 2 NOBr
Nitrosyl bromide is highly unstable and tends to decompose over time, especially in the presence of heat or light. It is a reactive compound and can react with various other chemicals. For example, it can react with water to form nitrous acid (HNO2) and hydrobromic acid (HBr).
Properties
- Chemical formula: NOBr
- Molar mass: 109.910 g/mol
- Appearance: Red gas
- Boiling point: 14.5 °C (58.1 °F; 287.6 K)
Application
NOBr is largely employed in chemical research and is not commonly used in industrial or commercial applications. It has been investigated for its reactivity and as a source of nitrosonium ions (NO+), which are required in certain chemical reactions. It is a helpful reagent in organic synthesis and is occasionally used in analytical chemistry.
Toxicity
Nitrosyl bromide is hazardous and should be handled with caution. Inhalation or skin contact might be hazardous to one’s health. Because of its toxicity and reactivity, it should only be handled and worked with by trained individuals in a controlled laboratory environment, following proper safety procedures and guidelines.