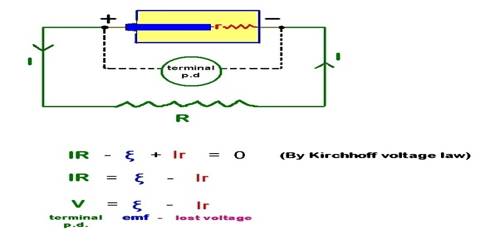
Experiment: Under what condition the potential difference of a cell becomes greater than the electromotive force of the cell?
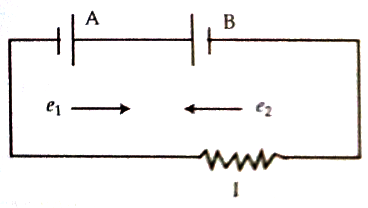
Normally, the electromotive force of a cell becomes greater than the potential difference between terminals of the cell. Electromotive force = potential difference + internal potential drop inside the cell; but if two cells of difference electromotive forces are connected in opposition, then the cell having higher electromotive force will charge the other cell i.e., current that the cell of less electromotive force usually allows to flow on its own, now the cell having higher electromotive force allows to flow charges through the other cell and consequently potential drop across this cell becomes greater than its electromotive force. According to figure, a cell having an electromotive force of e1 has been connected in opposition with another cell of the electromotive force of e2 (e2 > e1). In this case, the cell B will charge in the cell A. Potential drop between the terminals of the cell A will be greater than e1.
The terminal potential difference is the potential difference between the two electrodes of the cell in a closed circuit (ie. when current is drawn from the cell). The total potential difference in a circuit is always equal to the total electromotive force, not smaller. But real-world sources always have internal losses, and impedances so they often, not always, show a voltage smaller than the EMF at their terminals.