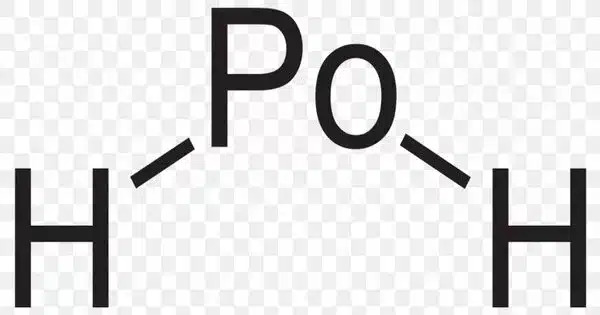
Polonium hydride is a compound made up of polonium and hydrogen. PoH2 is the formula for a chemical compound. It is a liquid at room temperature, the second hydrogen chalcogenide after water to have this property. It is chemically very unstable and decomposes into elemental polonium and hydrogen. It is a highly volatile and labile compound from which many polonides can be synthesized. Furthermore, it is radioactive.
Polonium hydride is formed when polonium reacts with hydrogen. It is a radioactive element that belongs to the periodic table’s chalcogen group (group 16). Because of their radioactivity and scarcity, polonium compounds have received little attention, and knowledge about them is limited. However, because polonium is scarce and there are safety concerns about its radioactivity, detailed experimental data on the properties of polonium hydride are limited.
Properties
Polonium hydride is a more covalent compound than most metal hydrides because polonium straddles the border between metals and metalloids and has some nonmetallic properties. It is an intermediate between a hydrogen halide like hydrogen chloride and a metal hydride like stannane.
- Chemical formula: PoH2
- Molar mass: 210.998 g/mol
- Melting point: −35.3 °C (−31.5 °F; 237.8 K)
- Boiling point: 36.1 °C (97.0 °F; 309.2 K)
Radioactivity
Polonium is well-known for its high radioactivity. One of its isotopes, polonium-210, undergoes alpha decay. If proper precautions are not taken, the decay process produces alpha particles, which are helium nuclei and can be harmful.
Chemical Reactivity
Polonium is a metal that, like other metals, can react with nonmetals such as hydrogen to form compounds. The chemical reactivity of polonium hydride is determined by the conditions in which it is formed.
Stability
Because of polonium’s radioactivity, any hydride formed may be highly unstable. The breakdown of chemical bonds within a compound can be aided by radioactive decay processes.
Preparation
Polonium hydride cannot be produced directly from the elements when heated. Other failed synthesis routes include the reaction of polonium tetrachloride (PoCl4) with lithium aluminum hydride (LiAlH4), which yields only elemental polonium, and the reaction of hydrochloric acid with magnesium polonide (MgPo). The failure of these synthesis routes could be attributed to radiolysis of polonium hydride during formation.
Reacting hydrochloric acid with polonium-plated magnesium foil yields trace amounts of polonium hydride. Furthermore, the formation and migration of polonium hydride may be responsible for the diffusion of trace amounts of polonium in palladium or platinum that is saturated with hydrogen.