A highly effective supercapacitor has been developed by a team collaborating with Roland Fischer, Professor of Inorganic and Metal-Organic Chemistry at the Technical University of Munich (TUM). The foundation of the energy storage unit is a novel, efficient, and also renewable hybrid graphene material with comparable performance data to the batteries currently in operation. Energy storage is typically connected with batteries and accumulators that provide energy to mobile devices. However, so-called supercapacitors are gradually being used in computers, cameras, cellphones, or cars these days.
The team working with TUM chemist Roland Fischer has now developed a novel, powerful as well as sustainable graphene hybrid material for supercapacitors.
Unlike batteries, they can easily store vast quantities of energy and bring it back almost as fast. When, for example, a train brakes before approaching the station, the supercapacitors store the energy and supply it again when the train needs a lot of energy very soon when it starts. One concern with the supercapacitors to date, though, was their lack of energy density. While lithium accumulators reach an energy density of up to 265 Kilowatt-hours (KW/h), supercapacitors thus far have only been delivering a tenth thereof.
Sustainable material provides high performance
The team working with TUM chemist Roland Fischer has now produced a novel, efficient, and renewable graphene hybrid material for supercapacitors. It is used as a positive electrode in the energy storage system. The researchers combined it with a known negative electrode built on titanium and carbon.
The current energy storage technology not only produces an energy density of up to 73 Wh/kg, which is approximately equal to the energy density of the nickel-metal hydride battery but also works significantly better than most other supercapacitors at a power density of 16 kW/kg. The key of the modern supercapacitor is the mixture of various materials, so chemists refer to the supercapacitor as “asymmetrical.”
Hybrid materials: Nature is the role model
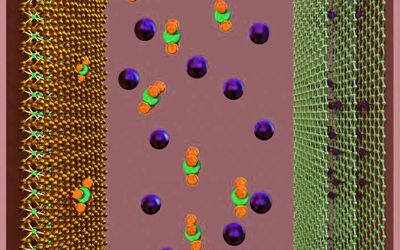
Researchers are banking on an alternative approach to solve the efficiency limitations of traditional materials – using composite materials. “Nature is filled with extremely complex, evolutionarily optimized composite materials – bones and teeth are evidence of this. Their mechanical characteristics, such as hardness and elasticity, have been optimized by the integration of different types of materials,” says Roland Fischer. The research team moved the abstract concept of mixing simple materials to the supercapacitors. As a basis, they used the novel positive electrode of the chemically engineered graphene storage unit and merged it with the nano-structured organic metal frame, the so-called MOF.
Powerful and stable
Decisive for the efficiency of graphene hybrids are, on the one hand, large specific surfaces and controllable pore sizes and, on the other hand, high electrical conductivity. “The high-performance capabilities of the material are based on the combination of microporous MOFs and conductive graphene acid,” describes the first reviewer, Jayaramulu Kolleboyina, a former guest scientist working with Roland Fischer.
A broad surface is very important for good supercapacitors. It allows a vast number of charge carriers to be collected inside the material – which is the fundamental concept for the conservation of electrical energy. The researchers have accomplished the feat of binding graphene acid to MOFs through the skillful nature of the content. The resulting hybrid MOFs have a very large inner surface of up to 900 square meters per gram and are highly efficient as positive electrodes in a supercapacitor.
Long stability
However, this is not the only advantage of the new content. In order to produce a chemically stable hybrid, a strong chemical bond between the components is required. The bonds are apparently the same as those between amino acids in proteins, according to Fischer: “In fact, graphene acid is linked to MOF-amino acid, which creates a type of peptide bond.”
The stable relationship between the nano-structured components has tremendous benefits in terms of long-term stability: the more stable the relationship, the more charging and discharging cycles are possible without substantial performance impairment. For comparison: the classic lithium accumulator has a useful life of around 5,000 cycles. The new cell produced by TUM researchers maintains almost 90% of its potential, even after 10,000 cycles.
The international network of experts
Fischer underlines the importance of the unfettered international collaboration of the researchers in the production of the modern supercapacitor. That’s why Jayaramulu Kolleboyina developed the squad. He was a guest scientist from India, invited by the Alexander von Humboldt Foundation, and now head of the chemistry department at the newly founded Indian Institute of Technology in Jammu.
“Our team also has a network of electro-chemical and battery research experts in Barcelona, as well as graphene-derived experts from the Czech Republic,” notes Fischer. “They already have interconnected partners from the USA and Australia. This fantastic foreign partnership guarantees a great deal for the future.”