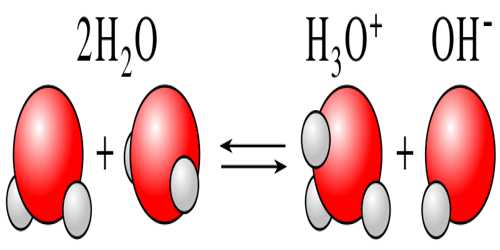
Self-Ionization (or autoionization) of Water
Water is known to be a non-electrolyte (non-conductor of electricity), although precise measurements indicate that water is a weak conductor of electricity. The conductivity of water arises due to a phenomenon is known as self-ionization. In water two molecules of water may react to give ions as shown below:
H2O (l) + H2O (l) ↔ H3O+ (aq) + OH– (aq)
The equilibrium constant for self-ionization of eater is given by,
K = {[H3O+] [OH–]} / [H2O]2 …. …. (1)
Since the concentration of the ions formed by self-ionization is very small, the concentration of H2O remains practically constant and equation (1) can be rewritten as,
[H2O]2 x K = [H3O+] x [OH–] …. …. (2)
The quantities on the left-hand side of equation (2) are constants and maybe, replaced by a new constant term KW, known as the ionic product of water.
KW = [H3O+] x [OH–] …. …. (3)

KW is a very important quantity of water. In all ionic equilibrium in water, KW is constant as long as the temperature is constant. In pure water
[H3O+] (or simply [H+]) = [OH–]
so that, KW = [H+]2 = [OH–]2 … …. …. (4)
The value of KW has been carefully determined at different temperatures by various methods. The value of KW at 298 K is about 1.0 x10-14 mol2 L-6. Whether the solution is acidic or alkaline the value of [H+] x [OH–] will always be 1.0 x 10-14 at 298 K. The ionic product KW of water may be calculated from conductance data as follows:
The specific conductance of pure water at 298 K is 5.50 x 10-8 ohm-1 cm-1. The ion conductances λ0H+ and λ0OH- at 298 K and at infinite dilutions are 349.8 cm2 ohm-1mol-1 and 198.0 cm2 ohm-1mol-1 respectively. So the molar conductance is-
Λ0 = λ0H+ + λ0OH-
= [349.8 cm2 ohm-1mol-1] + [198.0 cm2 ohm-1mol-1]
Using equation,
C = 1000k / Λ0 = (1000 x 5.5 x 10-8) / 547.8 = 1.00 x 10-7
where c is the concentration of ionized water, As each molecule of H2O gives one H+ ion and one OH– ion we can write;
c = [H+] = [OH–]
That is to say that in pure water the concentrations of H+ and OH– are equal.
Hence,
KW = [H+]2 = (1.00 x 10-7)2
= 1.0 x 10-14
The ionic product of water at various temperatures is given in Table.
Table: Values of KW at different temperatures