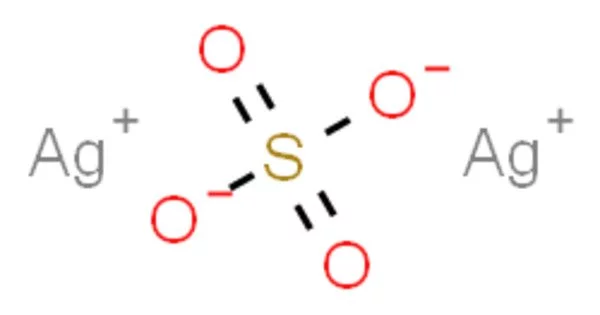
Silver sulfate (Ag2SO4) is an ionic compound that contains silver cations (Ag+) and sulfate anions (SO4 2-). It is a white crystalline solid that is insoluble in water. It is a strong oxidizing agent and can react with reducing agents to produce silver metal. It is stable under normal conditions, but can decompose at high temperatures to release sulfur trioxide gas. It can react with alkalis to form silver oxide, which is insoluble in water. It can react with halides to form insoluble silver halides.
Properties
- Appearance: white crystalline solid
- Molecular weight: 311.80 g/mol
- Melting point: 652°C (1206°F)
- Boiling point: decomposes at high temperatures
- Solubility: insoluble in water, soluble in concentrated sulfuric acid and in solutions of alkali cyanides
Preparation
Silver sulfate precipitates as a solid when an aqueous solution of silver nitrate is treated with sulfuric acid:
2 AgNO3 + H2SO4 → Ag2SO4 + 2 HNO3
It is purified by recrystallization from concentrated sulfuric acid, a step that expels traces of nitrate. Silver sulfate and anhydrous sodium sulfate adopt the same structure.
Synthesis of Silver(II) sulfate
The synthesis of silver(II) sulfate (AgSO4) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010 by adding sulfuric acid to silver(II) fluoride (HF escapes). It is a black solid that decomposes exothermally at 120 °C with evolution of oxygen and the formation of the pyrosulfate.
AgF2+H2SO4 →AgSO4 +2HF
Application
Silver sulfate is commonly used in analytical chemistry, particularly in the determination of halides (chlorides, bromides, and iodides) in a sample. This is because silver ions form insoluble silver halides with halide ions in solution, and the amount of silver halide formed can be used to determine the concentration of the halide ion.
- It is used in medicine as an antimicrobial agent
- It is commonly used in silver plating
- It is used in creams to treat wound infections
- It is used as an antibiotic
Silver sulfate is also used in the preparation of other silver compounds, such as silver nitrate, and as a catalyst in some organic reactions. It has some limited use in the photographic industry as a light-sensitive material. It is important to note that silver sulfate can be toxic if ingested or inhaled, so appropriate precautions should be taken when handling it.