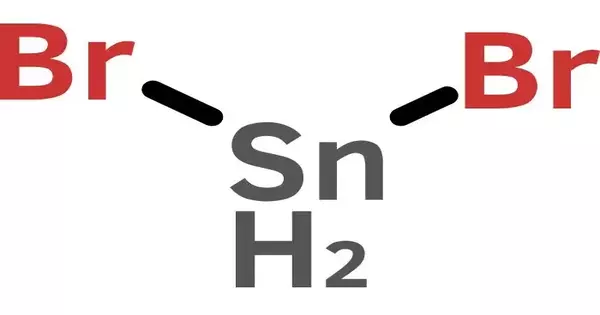
Tin(II) bromide is a tin bromide. It is a tin and bromine chemical compound with the formula SnBr2. Tin has an oxidation state of +2. The inert pair effect is responsible for the stability of tin compounds in this oxidation state.
Tin has the chemical symbol Sn and the atomic number 50. It is a naturally occurring component of the earth’s crust that is primarily obtained from the mineral cassiterite, where it occurs as tin dioxide.
Properties
- Chemical formula: SnBr2
- Molar mass: 278.518 g/mol
- Appearance: yellow powder
- Density: 5.12 g/cm3, solid
- Melting point: 215 °C (419 °F; 488 K)
- Boiling point: 639 °C (1,182 °F; 912 K)
- Crystal structure: related to PbCl2

Preparation
Tin(II) bromide can be prepared by the reaction of metallic tin and HBr distilling off the H2O/HBr and cooling:
Sn + 2 HBr → SnBr2 + H2
However, the reaction will produce tin (IV) bromide in the presence of oxygen.
Reactions
SnBr2 forms pyramidal adducts in donor solvents such as acetone, pyridine, and dimethylsulfoxide.
There are several hydrates known, including 2SnBr2•H2O, 3SnBr2•H2O, and 6SnBr2•5H2O, which have tin coordinated by a distorted trigonal prism of 6 bromine atoms with Br or H2O capping 1 or 2 faces in the solid phase. When SnBr3 is dissolved in HBr, the pyramidal ion SnBr3 is formed. It is a reducing agent like SnCl2. Oxidative addition can occur with a variety of alkyl bromides to produce alkyltin tribromide, for example.
SnBr2 + RBr → RSnBr3
Tin(II) bromide can act as a Lewis acid forming adducts with donor molecules e.g. trimethylamine where it forms NMe3·SnBr2 and 2NMe3·SnBr2. It can also act as both donor and acceptor in, for example, the complex F3B·SnBr2·NMe3 where it is a donor to boron trifluoride and an acceptor to trimethylamine.
Uses
It functions as a Lewis acid. It is used in the synthesis of alkyl tin compounds as well as the spectrophotometric determination of iridium. It participates in the regioselective mono-etherification of vicinal diols with diazo compounds. It acts as a catalyst in the formation of resinous lactone high polymers. It is a powerful reducing agent.