Two equal and opposite charges separated by a very small distance constitute an electric dipole.
Water, ammonia, carbon−dioxide and chloroform molecules are some examples of permanent electric dipoles. These molecules behave like electric dipole, because the centres of positive and negative charge do not coincide and are separated by a small distance.
Two point charges +q and –q are kept at a distance 2d apart (Figure). The magnitude of the dipole moment is given by the product of the magnitude of the one of the charges and the distance between them.
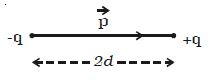
Electric dipole moment, p = q2d or 2qd.
It is a vector quantity and acts from –q to +q. The unit of dipole moment is C m.