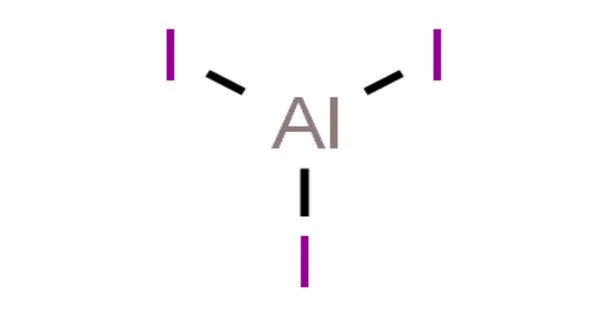
Aluminum iodide, also known as aluminium triiodide, is a chemical compound with the formula AlI3. It is an ionic compound that consists of positively charged aluminum ions (Al3+) and negatively charged iodide ions (I-). It is a white or yellowish powder that is soluble in organic solvents but insoluble in water.
Aluminium iodide is a chemical compound that contains the elements aluminum and iodine. The name always refers to an AlI3 compound formed by the reaction of aluminium and iodine or the action of HI on Al metal. A reaction between metallic aluminum or aluminum hydroxide and hydrogen iodide or hydroiodic acid produces hexahydrate. AlI3, like chloride and bromide, is a strong Lewis acid that absorbs water from the atmosphere. It is used as a reagent in the scission of certain C-O and N-O bonds. It deoxygenates epoxides and cleaves aryl ethers.
Properties:
- Appearance: It is a white, odorless powder.
- Melting and boiling point: The melting point is 192°C, and its boiling point is 380°C.
- Solubility: It is soluble in water, ethanol, and acetone.
- Stability: It is hygroscopic, which means it absorbs moisture from the air, and can decompose when exposed to air.
- Chemical properties: It is a Lewis acid, which means it can accept electron pairs from Lewis bases. It reacts with Lewis bases such as water and alcohols to form hydrated aluminum ions.
Preparation
Aluminum iodide is typically prepared by reacting aluminum with iodine in the presence of a catalyst, such as red phosphorus. It can also be prepared by the reaction of aluminum hydroxide with hydroiodic acid.
Application
Aluminum iodide is primarily used as a catalyst in organic synthesis, particularly in Friedel-Crafts reactions. It has a variety of uses, including as a catalyst in organic synthesis reactions, as a starting material for the synthesis of other aluminum compounds, and as a reagent in the manufacture of some types of batteries. It is also used in some nuclear reactors as a neutron absorber.
Health effects
Aluminum iodide is a strong irritant and can cause skin and eye irritation upon contact. It should be handled with care, and protective equipment should be worn when handling it.