Aluminium nitride (AlN) is a technological ceramic that does not include any oxides. It is a solid aluminum nitride. It is an electrical insulator and has a high thermal conductivity of up to 321 W/(m•K). It is well known for its strong thermal conductivity, resistance to high temperatures, and ability to act as an electrical insulator. As a result, it is frequently employed as a heat sink or as a substrate in electronics where high thermal conductivity is required, such as LEDs and semiconductors.
Its wurtzite phase (w-AlN) has a bandgap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies. AlN was first synthesized in 1862 by F. Briegleb and A. Geuther.
Physical properties
AlN, in the pure (undoped) state has an electrical conductivity of 10−11–10−13 Ω−1⋅cm−1, rising to 10−5–10−6 Ω−1⋅cm−1 when doped. Electrical breakdown occurs at a field of 1.2–1.8×105 V/mm (dielectric strength).
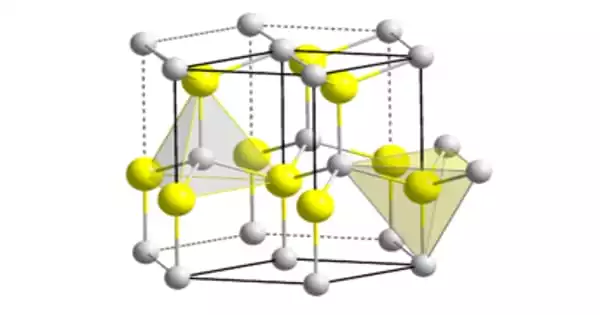
The material exists largely in the hexagonal wurtzite crystal structure, but it also possesses a metastable cubic zincblende phase that is primarily manufactured in the form of thin films. At high pressures, the cubic phase of AlN (zb-AlN) is expected to exhibit superconductivity.
A high-quality MOCVD-grown AlN single crystal has an intrinsic thermal conductivity of 321 W/(m•K), which is consistent with a first-principles calculation. It is 70–210 W/(m•K) for polycrystalline material and as high as 285 W/(m•K) for single crystals for an electrically insulating ceramic).
- Molecular Weight: 40.9882
- Appearance: White to pale yellow powder
- Melting Point: 2200 °C
- Boiling Point: 2517 °C (dec.)
- Density: 2.9 to 3.3 g/cm3
- Solubility in H2O: N/A
- Electrical Resistivity: 10 to 12 10x Ω-m
- Poisson’s Ratio: 0.21 to 0.31
- Specific Heat: 780 J/kg-K
- Thermal Conductivity: 80 to 200 W/m-K

Stability and chemical properties
The chemical formula for aluminium nitride is AlN. It is an inorganic covalently bonded chemical with a hexagonal wurtzite crystal structure. It has a density of 3.3 grams per cubic centimeter and a molar mass of 40.99 grams per mol.
Aluminium nitride is stable at high temperatures in inert atmospheres and melts at around 2200 degrees Celsius. At ~1800 °C, AlN decomposes in a vacuum. Surface oxidation occurs in the air over 700 °C, and surface oxide layers of 5–10 nm thickness have been detected at ambient temperature. This oxide layer protects the material from temperatures as high as 1370 °C. Above this temperature, mass oxidation takes place. Up to 980 °C, aluminium nitride is stable in hydrogen and carbon dioxide atmospheres.
The material dissolves slowly in mineral acids through grain boundary attack and in strong alkalies through an attack on the aluminum-nitride grains. The material hydrolyzes slowly in water. Aluminium nitride is resistant to attack from most molten salts, including chlorides and cryolite.
Aluminium nitride can be patterned with a Cl2-based reactive ion etch.
Applications
Aluminum nitride is particularly desirable as a substrate material due to the high temperatures involved in semiconductor manufacturing processes, as well as the sensitivity of the devices under development. Because of its high thermal conductivity, it can serve as a good heat sink while remaining electrically insulating and without breaking at high temperatures. Furthermore, it has a coefficient of thermal expansion that is quite comparable to silicon.
AlN is also employed in the fabrication of piezoelectric micromachined ultrasonic transducers that transmit and receive an ultrasound and can be used for in-air rangefinding across distances of up to a meter.
There are metallization technologies available to enable AlN to be employed in electronics applications similar to alumina and beryllium oxide. AlN nanotubes as inorganic quasi-one-dimensional nanotubes, which are isoelectronic with carbon nanotubes, have been suggested as chemical sensors for toxic gases.