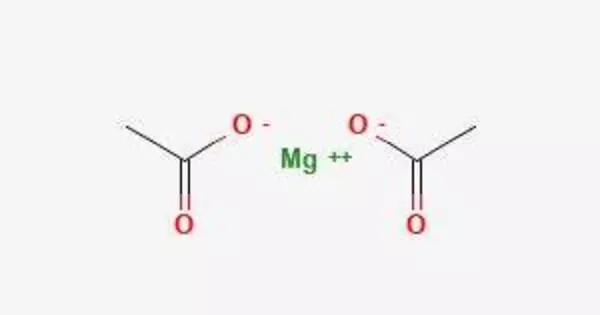
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2, and its hydrated form, magnesium acetate tetrahydrate, has the chemical formula Mg(CH3COO)2 • 4H2O. Magnesium has the oxidation state 2+ in this compound. It is the magnesium salt of acetic acid and is commonly used in a variety of industrial and laboratory applications.
Magnesium acetate is the acetic acid magnesium salt. It is deliquescent and, when heated, decomposes to form magnesium oxide. Magnesium acetate is commonly used as a magnesium source in biological reactions.
Properties
Magnesium acetate appears as white hygroscopic crystals. It smells like acetic acid and is soluble in water. When it is in an aqueous solution its pH will be on the alkaline side of neutral.
- Chemical formula: Mg(CH3COO)2
- Molar mass: 142.394 (anhydrous) 214.455 (tetrahydrate)
- Appearance: White hygroscopic crystals
- Density: 1.45 g/cm3 (tetrahydrate)
- Melting point: 80 °C (176 °F; 353 K) (tetrahydrate)
- Solubility in water: Soluble
Preparation
Anhydrous magnesium acetate can be prepared by reacting magnesium oxide or magnesium hydroxide with acetic acid. The reaction forms water as a byproduct.
Mg(OH)2 +2CH3COOH → Mg(CH COO)2 +2H2O
Storage
Due to the fact that it is very hygroscopic, it must be stored away from water. It is also incompatible with strong oxidizers and should not be mixed with them.
Applications
- Deicing Agent: It is used as a deicing agent on roads and sidewalks. It is less corrosive than some other deicing salts, making it a preferred choice in certain situations.
- Catalysis: It is used as a catalyst in various chemical reactions.
- Desiccant: Due to its hygroscopic nature, it can be used as a desiccant to absorb moisture.
Safety Precautions
As with handling any chemical compound, it is important to follow proper safety precautions. This includes using personal protective equipment such as gloves and goggles.