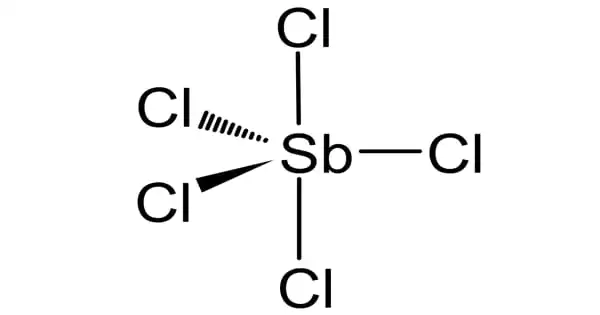
Antimony pentachloride is a chemical compound having the formula SbCl5. It is a liquid that looks as a reddish-yellow fuming liquid with a strong stench. It is a colorless oil, however normal samples are yellowish due to dissolved chlorine. SbCl5 is a very caustic material that must be maintained in glass or PTFE containers due to its potential to hydrolyze to hydrochloric acid.
It is employed in the analysis of caesium and alkaloids, dyeing, and as an intermediate in chemical synthesis. It is also a powerful oxidizing agent. Antimony pentachloride inhalation produces nose and throat discomfort. Contact with the eyes or skin results in serious burns. Ingestion results in vomiting and severe burns to the mouth and stomach. Overexposure by any route can cause bloody stools, slow pulse, low blood pressure, coma, convulsions, and cardiac arrest.
Properties
It is colorless in nature but due to the impurities mixed in it appears to be reddish yellow or dark brown in color. It is found in an oil-like state and is highly corrosive in nature. It is a powerful oxidizing agent. Its inhalation produces nose and throat discomfort.
- Chemical formula: Cl5Sb
- Molar mass: 299.01 g·mol−1
- Appearance: colorless or reddish-yellow (fuming) liquid, oily
- Odor: pungent, offensive
- Density: 2.336 g/cm3 (20 °C); 2.36 g/cm3 (25 °C)
- Melting point: 2.8 °C (37.0 °F; 275.9 K)
- Boiling point: 140 °C (284 °F; 413 K); decomposes from 106 °C; at 22 mmHg
- Solubility in water: reacts
- Solubility: soluble in alcohol, HCl, tartaric acid, CHCl3, CS2, CCl4

Preparation and structure
Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride:
SbCl3 + Cl2 → SbCl5
Gaseous SbCl5 has a trigonal bipyramidal structure.
Reactions
This compounds reacts with water to form antimony pentoxide and hydrochloric acid:
2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl
The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O.
This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity.
It is also a strong oxidizing agent. For example aromatic ethers are oxidized to their radical cations according to the following stoichiometry:
3 SbCl5 + 2 ArH → 2 (ArH+)(SbCl6–) + SbCl5
Applications
Antimony pentachloride is used as a polymerization catalyst and for the chlorination of organic compounds.
- It is used as a catalytic and analytical agent for testing alkaloids.
- It is used during the chlorination of organic compounds.
- It is also used as a strong oxidizing agent or Lewis acid.
- It is used as a catalyst while replacing fluorine with chlorine in organic compounds.
Precautions
Antimony pentachloride is a very corrosive chemical that should be kept cool and dry. It is a chlorinating agent that emits hydrogen chloride gas when exposed to moisture. As a result, if handled in a damp environment, it may etch even stainless-steel implements (such as needles). Because it melts and carbonizes non-fluorinated polymers (such as plastic syringes, plastic septa, or needles with plastic fittings), it should not be used with them.