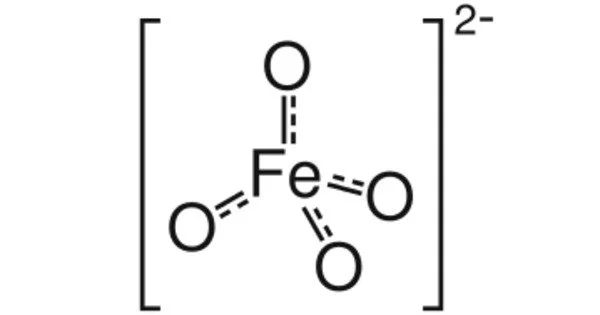
The chemical compound barium ferrate has the formula BaFeO4. This is a rare iron compound in the +6 oxidation state. The ferrate(VI) ion is paramagnetic because it has two unpaired electrons. It is structurally similar to BaSO4 and contains the tetrahedral anion [FeO4]2-.
Barium is a metallic alkaline earth metal with the atomic number 56 and the symbol Ba. Due to its reactivity with air, it never occurs in its pure form in nature, but instead combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds, which can be found as minerals.
Properties
It is a pinkish-brown powder that is insoluble in water and other solvents. Although it is a strong oxidizer, its insolubility makes it extremely stable for a ferrate. It is not known to decompose simply by coming into contact with air, as potassium ferrate frequently does.
- Chemical formula: BaFeO4
- Molar mass: 257,1646 g/mol
- Appearance: Dark red, opaque crystals
- Solubility in water: insoluble
- Crystal structure: orthorhombic

Preparation
Barium ferrate(VI) can be prepared by both wet and dry synthetic methods. Dry synthesis is usually performed using a thermal technique, such as by heating barium hydroxide and iron(II) hydroxide in the presence of oxygen to about 800 to 900 °C.
Ba(OH)2 + Fe(OH)2 + O2 → BaFeO4 + 2 H2O
Wet methods employ both chemical and electrochemical techniques. For example, the ferrate anion forms when a suitable iron salt is placed in alkaline conditions and a strong oxidising agent, such as sodium hypochlorite, is added.
2 Fe(OH)3 + 3 OCl– + 4 OH– → 2 FeO42- + 5 H2O + 3 Cl–
The precipitation of barium ferrate from solution is then accomplished by adding a solution of a barium(II) salt. When a soluble barium salt is added to an alkali metal ferrate solution, it produces a maroon precipitate of barium ferrate, a crystal with the same structure and solubility as barium chromate. At room temperature (or 0 °C), barium ferrate was also prepared by adding barium oxide to a mixture of sodium hypochlorite and ferric nitrate. The purity of the product can be increased by performing the reaction at a low temperature in the absence of carbon dioxide and rapidly filtering and drying the precipitate, which reduces the coprecipitation of barium hydroxide and barium carbonate as impurities.
It, however, reacts with acids stronger than ferric acid, such as all strong acids and several mid-strength acids such as phosphoric, liberating the extremely unstable ferric acid which is a powerful oxidizer.
Uses
Barium ferrate is an oxidizing agent that is used as an oxidizing reagent in organic synthesis. Its other applications include color removal, cyanide removal, bacteria killing, and contaminated and waste water treatment.
Ferrate(VI) salts are high-energy cathode materials in “super-iron” batteries. Cathodes containing ferrate(VI) compounds are referred to as “super-iron” cathodes due to their highly oxidized iron basis, multiple electron transfer, and high intrinsic energy. Among all ferrate(VI) salts, barium ferrate has an unusually easy charge transfer, which is important for the high power domain of alkaline batteries.
Reactions
Barium ferrate is the most stable of the ferrate(VI) compounds. It can be prepared in its purest form and has the most precise composition. All soluble acids, including carbonic acid, can easily degrade barium ferrate. If carbon dioxide is passed through water containing hydrated barium ferrate, the barium ferrate completely decomposes to form barium carbonate, ferric hydroxide, and oxygen gas. Alkaline sulfates decompose untreated barium ferrate, producing barium sulfate, ferric hydroxide, and oxygen gas.