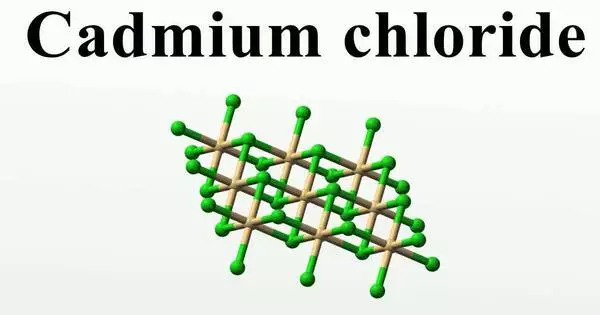
Cadmium chloride, abbreviated CdCl2, is a white crystalline compound of cadmium and chloride. It is a whitish, crystalline inorganic compound that, when heated, emits toxic cadmium oxide fumes. This salt is a hygroscopic solid that is highly soluble in water but only moderately soluble in alcohol. Cadmium chloride’s crystal structure serves as a model for describing other crystal structures. CdCl2•H2O and CdCl2•5H2O are other names for this compound.
Cadmium chloride is used in electroplating, printing, photocopying, dyeing, mirrors, vacuum tubes, lubricants, analytical chemistry, and as a chemical intermediate in the production of cadmium pigments and stabilizers.
Properties
Cadmium chloride is a crystalline white solid. It’s an odorless and colorless crystal. It dissolves in water. It is not flammable. The primary danger of this material is that it is hazardous to the environment. Immediate action should be taken to limit its environmental spread.
- Chemical formula: CdCl2
- Molar mass: 183.31 g·mol-1
- Appearance: White solid, hygroscopic
- Odor: Odorless
- Density: 4.047 g/cm3 (anhydrous); 3.327 g/cm3 (Hemipentahydrate)
- Melting point: 568 °C (1,054 °F; 841 K) at 760 mmHg
- Boiling point: 964 °C (1,767 °F; 1,237 K) at 760 mmHg
- Solubility: Soluble in alcohol, selenium(IV) oxychloride, benzonitrile
- Insoluble in: ether, acetone
- Crystal structure: Rhombohedral, hR9 (anhydrous)

Structure
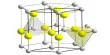
Cadmium chloride forms a layered structure consisting of octahedral Cd2+ centers linked with chloride ligands. Cadmium iodide, CdI2, has a similar structure, but the iodide ions are arranged in a HCP lattice, whereas in CdCl2 the chloride ions are arranged in a CCP lattice.
Chemical properties
Cadmium chloride dissolves well in water and other polar solvents. It is a mild Lewis acid.
CdCl2 + 2 Cl− → [CdCl4]2-
Solutions of equimolar cadmium chloride and potassium chloride give potassium cadmium trichloride. With large cations, it is possible to isolate the trigonal bipyramidal [CdCl5]3- ion. It is incompatible with bromine trifluoride, potassium oxidisers, zinc, selenium, tellurium, and hydrogen azide.
Preparation
Anhydrous cadmium chloride can be prepared by the reaction of hydrochloric acid and cadmium metal.
Cd + 2 HCl → CdCl2 + H2
The anhydrous salt can also be prepared from anhydrous cadmium acetate using hydrogen chloride or acetyl chloride.
Uses
Cadmium chloride is used to make cadmium sulfide, which is then used to make “cadmium yellow,” a brilliant-yellow stable inorganic pigment. It is used in photography, fabric printing, chemical analysis, and a variety of other applications.
Cadmium chloride is also used in the photocopying, dyeing, and electroplating processes. CdCl2, like all cadmium compounds, is extremely toxic and must be handled with extreme caution.
Health Effect
This substance irritates the eyes, skin, and respiratory tract, damages the lungs, causing shortness of breath, chest pain, and pulmonary edema, and can also damage the kidneys, causing proteinuria and decreased renal function. Cadmium chloride is a known carcinogen that has been linked to an increased risk of developing lung cancer.