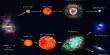
Chemical Weathering Processes
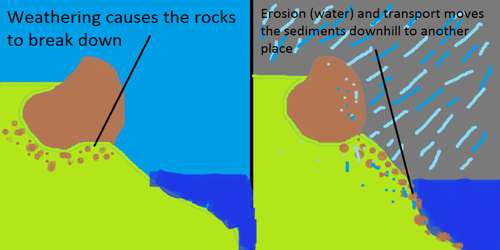
A group of weathering processes viz; solution, carbonation, hydration, oxidation, and reduction act on the rocks to decompose, dissolve or reduce them to a fine elastic state through chemical reactions by oxygen, surface and/or soil water and other acids. Water and air (oxygen and carbon dioxide) along with heat must be present to speed up all chemical reactions. Over and above the carbon dioxide present in the air, decomposition of plants and animals increases the quantity of carbon dioxide underground. These chemical reactions on various minerals are very much similar to the chemical reactions in a laboratory.

Solution
When something is dissolved in water or acids, the water or acid with dissolved contents is called solution. This process involves removal of solids in solution and depends upon the solubility of a mineral in water or weak acids. On coming in contact with water many solids disintegrate and mix up as a suspension in water. Soluble rock-forming minerals like nitrates, sulphates, and potassium etc. are affected by this process.
Carbonation
Carbonation is the reaction of carbonate and bicarbonate with minerals and is a common process helping the breaking down of feldspars and carbonate minerals. Carbon dioxide from the atmosphere and soil air is absorbed by water, to form carbonic acid that acts as a weak acid. Calcium carbonates and magnesium carbonates are dissolved in carbonic acid and arc removed in a solution without leaving any residue resulting in cave formation.
Hydration
Hydration is the chemical addition of water. Minerals take up water and expand; this expansion causes an increase in the volume of the material itself or rock. Calcium sulphate takes in water and turns to gypsum, which is more unstable than calcium sulphate. This process is reversible and long, continued repetition of this process causes fatigue in the rocks and may lead to their disintegration.