Internal Energy:
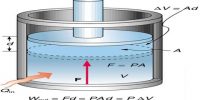
In thermodynamics the total energy possessed by a system is termed its internal energy (U). Internal energy is the sum of all possible forms of energy contained in a body. Internal energy of a system is a function of temperature, chemical nature of the system and sometimes also pressure and volume of the system. In a given system the magnitude of the internal energy depends on the way the molecules are put together and the nature of the individual atoms. The energy possessed by the molecules, the arrangement and number of electrons in the molecules, all contribute to U. In addition, the energy due to the vibrational, rotational and translational motions of the molecules is also included in U. Chemical and physical changes are accompanied by rearrangements in the relative positions of the atoms. This leads to changes in internal energy.
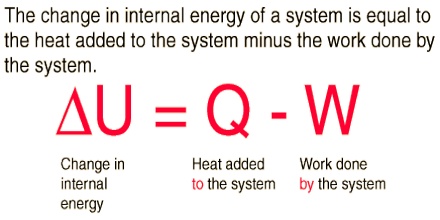
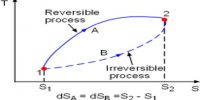
At the present time it is impossible to determine the absolute value of the internal energy of a system. Fortunately, however, it is the change in internal energy accompanying a chemical or physical change that is of interest to chemists. Absorption or releases of heat which accompany chemical or physical changes are manifestations of the change in internal energy. The difference between the internal energy when a system changes from state A to state B is expressed as ∆U, so that;
∆U = UB – UA
where, UB is the internal energy to the final state and UA is the energy in the initial state. The symbol ∆ always indicates the difference between the values of the property of a system in the final state and in the initial state.