Rusting of iron consists of the formation of hydrated oxide. Look at the following two half equations:
Fe2+ (aq) + 2e– → Fe(s) E° = -0.44 V
2H2O (I) + 2e– → H2 ÷ OH– (aq) E° = -0.42 V
Will the reaction between Fe and H2O proceed? The reaction will proceed spontaneously, but with only a small emf. However, if oxygen is also present, another reaction can also take place:
O2 (g) + 4H+ (aq) + 4e– → 2H2O E° = +0.82 V
This reaction has a sufficiently positive emf to readily oxidise the Fe to Fe2+, and then consequently to Fe3+. The final product is a brown/orange compound of formula Fe2O3.x H2O, which is commonly referred to as rust.
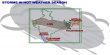
Rusting is the cause of extensive corrosion of iron pipelines, tanks, buildings etc, which is difficult and expensive to repair. Thus, iron and steel need to be protected by painting, greasing or galvanising, so that the oxygen is not allowed in contact with the iron. Alternatively, a piece of a more reactive metal, such as magnesium is attached at a convenient place. This will form a voltaic cell, in which the magnesium forms the anode, and the iron acts as the cathode. Thus the magnesium, rather than the iron, will be oxidised – and can simply be replaced when used up. This use of an active metal is called cathodic (or sacrificial) protection.