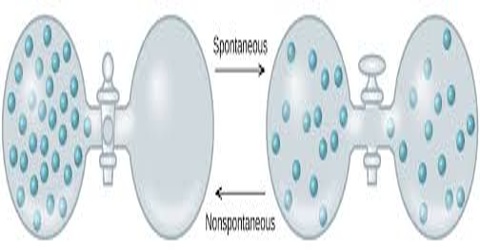
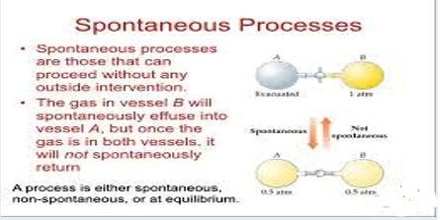
Spontaneous Process:
A process that takes place without any outside influence is called a spontaneous process. A spontaneous process is capable of proceeding in a given direction without needing to be driven by an outside source of energy. All natural processes are spontaneous. Rivers run from the mountains to the sea; water freezes at or below 0°C; trees bear fruit and then die; heat flows from a hot body to a cooler-body; a dissolved solute diffuses from a region of high concentration to a region of low concentration; a gas expands from high pressure to a low pressure. All these are examples of spontaneous processes that take place in nature without any outside influence.
The spontaneous natural processes are used by man to obtain useful work. Thus, in a hydroelectric project the falling water turns the blades of the generator which produces electricity; an expanding gas pushes the piston in a motor car or a steam engine. Spontaneous processes are abundant in nature. It may be observed that as a result of a spontaneous process a system loses its ability to do work. Hence although the total energy of the universe remains constant its availability diminishes.
Spontaneous processes are not reversible as at no stage of the process equilibrium is obtaining except at the final stage; in other words natural processes are irreversible. In a reversible process the system is at equilibrium at all stages so that an infinitesimal increase in the tendency opposing the process will bring about its reversal.