Hess’s law is known as after Russian Chemist Germain Hess. Hess helped formulate the early standards of thermochemistry. His famous paper, which was published in 1840, included his law.
Hess’s law: If the initial and final condition remains constant the chemical reaction occurs at one step or more than one steps, in every step the reaction enthalpy will be equal. This is also known as the law of constant heat summation.
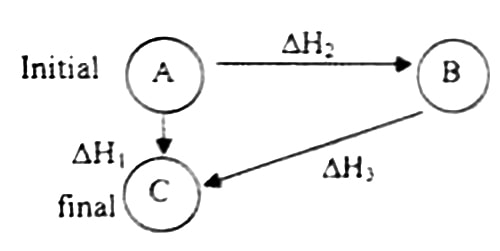
According to Hess’s law, ∆H1 = ∆H2 + ∆H3
So, Hess’s law states that energy changes are state functions. The amount of energy depends only on the states of the reactants and the state of the products, but not on the intermediate steps. Eenthalpy changes in chemical reactions are the same, regardless whether the reactions occur in one or several steps.