Foam
A dispersion of a gas in a liquid is called Foam. Ordinary soap solutions form lather which behaves like a foam if air-bubbles entrapped into it is reduced to colloidal dimension.
Sol, gel, emulsion, and foam are important colloids which find uses in our daily life.
Foams are examples of isolated media. In general, gas is present, so it divides into gas bubbles of dissimilar sizes (i.e., the substance is polydisperse)—alienated by fluid regions that may form films, thinner and thinner when the fluid phase drains out of the system films. When the major scale is miniature, i.e., for a very fine foam, this dispersed medium can be considered a sort of colloid.
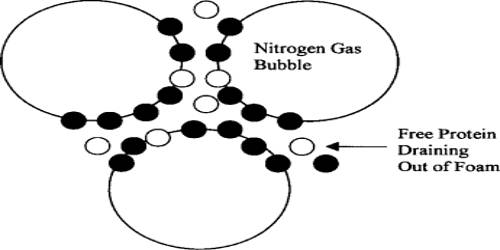
Foam can also refer to something that is analogous to foam, such as quantum foam, polyurethane foam (foam rubber), XPS foam, polystyrene, phenolic, or any other artificial foams. The foam used to combat oil fires consists of bubbles of carbon dioxide (liberated from sodium bicarbonate and aluminum sulfate) stabilized by dried blood, glue, or other cheap protein-containing materials. Beer foam is believed to be stabilized by the colloidal constituents present, which contain proteins and carbohydrates.